The demand for replacing degenerated bioprosthetic valves (BPVs) is steadily rising owing to the increasing burden of heart disease, ageing populations, advances in surgical intervention and limited durability of current-generation surgical BPVs. The issue is exacerbated by the increasing trend of surgical BPV replacement, especially in younger patients who are likely to require future intervention.1
Transcatheter aortic valve implantation (TAVI) is a safe and effective alternative to surgical aortic valve replacement for patients across the entire risk spectrum, although the importance of the heart team in clinical decision-making remains paramount.2 Structural valve degeneration has bedevilled surgical aortic valve replacement since its inception, and studies have shown high mortality rates associated with redo surgical aortic valve replacement, estimated between 3.8% and 5.2%. Since the first valve-in-valve (VIV) TAVI procedure was performed by Wenaweser et al. in 2007, there is now a less invasive option to treat patients with degenerated BPVs.3 Patient prosthesis mismatch (PPM) following VIV TAVI, however, has emerged as a concern, particularly among patients with smaller BPVs. Bioprosthetic valve fracturing (BVF) of a BPV during VIV TAVI is a novel approach, first described in the aortic position in 2015, involving fracturing the BPV ring with high-pressure balloon inflation to improve postprocedural valvular haemodynamics.4
Valve-in-valve Transcatheter Aortic Valve Implantation
The Valve-in-Valve International Data (VIVID) registry, established in 2010, was designed to collect data on VIV TAVI procedures using both self-expanding (Medtronic) and balloon-expandable (Edwards Lifesciences) devices. It is the largest of its kind.5 The VIVID registry showed worse outcomes when VIV TAVI was performed in patients with small surgical valve sizes (label size ≤21 mm) and in those with stenosis as the primary mechanism of failure.5
Small surgical valve size (≤21 mm) was associated with a reduced 1-year survival of 74.8% compared with intermediate-sized (21–25 mm) and large (≥25 mm) BPVs, which had survival rates of 81.8% and 93.3%, respectively.5 Patients being treated for BPV stenosis had 30-day mortality rates of 10.5% compared with 4.3% for BPV regurgitation and 7.2% in the combined group (p=0.04).5 One multicentre study (n=47) describes a 30-day mortality rate of 17% after ViV TAVI (mean age 80.3 years; EuroSCORE 35%).6
PPM is assumed to be the cause of increased mortality rates after VIV TAVI and as such is a major concern.5 PPM is generally defined as an indexed effective orifice area (iEOA) ≤0.85 cm2/m2 and can lead to an inadequate cardiac index.7 In one series, PPM was highest among VIV TAVI within smaller BPVs and the incidence of severe PPM (iEOA ≤0.65 cm2/m2) following VIV TAVI was found to be 31.8 %.5 If, however, one considers that, for example, Mitroflow 19 mm and 21 mm prostheses have true internal diameters of 15.4 mm and 17.3 mm, respectively,5 it is perhaps not surprising that placement of a THV constrained by these diameters may not meet physiological demands. In summary, patients with small surgical BPVs undergoing VIV TAVI have higher residual gradients and higher mortality compared with patients with larger BPVs undergoing VIV TAVI.5
Bioprosthetic Valve Fracture
Owing to the poorer outcomes among patients undergoing ViV TAVI with smaller diameter BPVs (i.e. 19 mm and 21 mm BPVs), several investigators have begun to consider interventions that might reduce the incidence of PPM. BVF was first described in the aortic position by Nielsen-Kudsk et al. in 2015.4 The procedure involves fracturing the ring of the structural ring of the surgical BPV using a non-compliant balloon during rapid ventricular pacing to allow for greater expansion of the transcatheter heart valve (THV) and possibly the implantation of a larger THV size more appropriate to the patient’s cardiac index.4,6–14 At present, it remains unclear as to whether it is the complete expansion of the THV or the implantation of a larger valve that is the main factor contributing to the improved haemodynamics found after BVF.
Bioprosthetic Valve Fracturing Procedure and Case Series
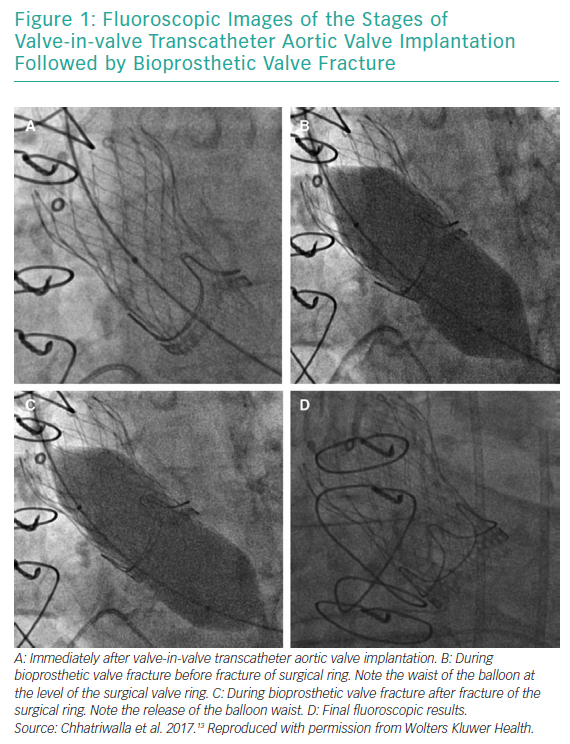
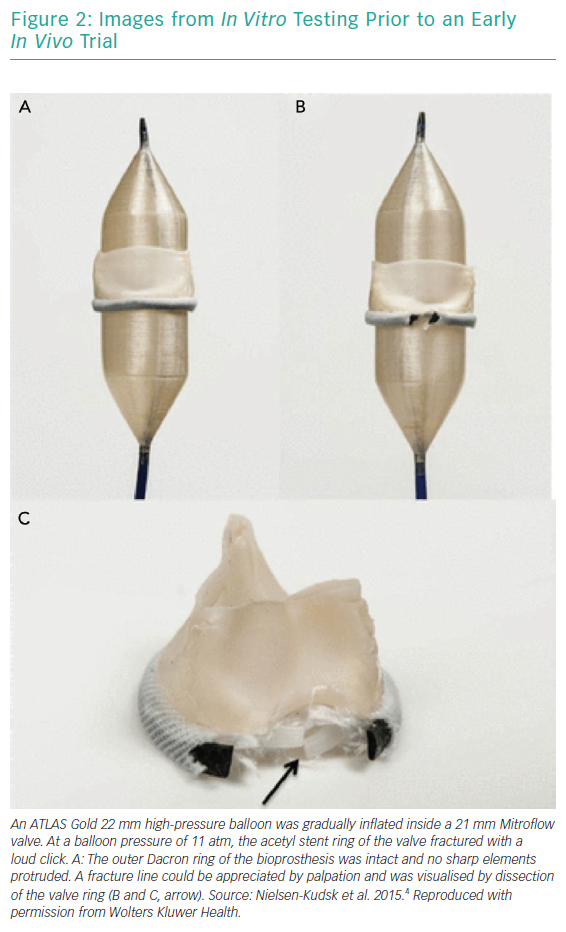
BVF fracture directly addresses the issue of inadequate valve diameter by forcefully dismantling the rigid scaffold of the degenerated surgical BPV (Figures 1 and 2). This allows the implantation of a valve size better suited to the patient’s native cardiac index. While the composition of BPVs vary, all are based on similar concepts. For example, the design of the Mitroflow BPV (Sorin Group; Figure 2) incorporates a bovine pericardial sheet sutured to the outside of an acetyl stent to form the leaflets.4 The sewing ring is made from soft radiopaque silicone covered by a Dacron mesh. The acetyl stent ring diameter can be widened via fracturing.4
Bench tests describe the technique of placing a non-compliant balloon within the surgical bioprosthesis.4,9–11 A high-pressure stopcock is used to separately attach a syringe and an indeflator to the balloon. The balloon is inflated by hand injection at first, and is then completed with high-pressure inflation using the indeflator.9 This results in a single fracture point within the stiff valve ring. An audible snap can be heard with a sudden decrease in inflation pressure. The fracture can usually be confirmed visually by fluoroscopy, but not in Mosaic (Medtronic) and Mitroflow BPVs, because they have no metal ring.12 Haemodynamic measurements and calculation of the valve effective orifice area are performed at baseline, immediately after VIV TAVI and after BVF.
Two single-centre case series demonstrate the strategies and haemodynamic results in patients treated with VIV TAVI and BVF.13,14 The majority of these cases were to treat stenotic BPVs, with a mean age of 79 years. Of a combined cohort of 30 patients from the two cases series, 15 patients underwent BVF before TAVI and the other 15 received BVF after TAVI.13,14 This has been clearly summarised in a prior review.12
Interestingly, the 15 cases of TAVI prior to BVF were all in one case series, and allow measurement of haemodynamic improvements following TAVI to be compared with further improvements post BVF.13 In this study, there were initial haemodynamic improvements observed following VIV TAVI, but importantly, further benefits were observed in the same patients following BVF. Mean transvalvular gradients were reduced from 20.5 ± 7.4 mmHg after initial VIV TAVI to 6.7 ± 3.7 mmHg after BVF (p<0.001).13 Accordingly, the mean effective orifice area increased from 1.0 ± 0.4 cm2 after initial VIV TAVI to 1.8 ± 0.6 cm2 after BVF (p<0.001).13
A multicentre case series in which BVF was performed in 75 patients in 21 centres has recently been published.8 BVF success was defined as when the waist of the balloon released and/or there was a sudden drop in inflation pressure on the indeflator. The outcomes corroborate previous observational studies, and suggest that BVF can be safely performed with balloons ≥3 mm larger than the BPV true internal diameter, thereby achieving significant reductions in transvalvular gradients.8 The series also recommended BVF be performed after VIV TAVI, as this sequence resulted in significantly lower mean gradients as compared with BVF performed before VIV TAVI (8.1 ± 4.8 mmHg versus 16.9 ± 10.1 mmHg; p<0.001).8 The most significant predictive factors for BVF success were timing of BVF (i.e. post-VIV TAVI BVF) and the use of balloon sizes ≥3 mm larger than the BPV true internal diameter.8
Surgical Bioprosthetic Valve Types
In the case series by Chhatriwalla et al. (n=20), both balloon-expandable (n=8) and self-expanding (n=12) THVs were used in Mitroflow (Sorin, Milan, Italy), Carpentier-Edwards Perimount, Magna and Magna Ease (Edwards Lifesciences), Biocor Epic and Biocor Epic Supra (St Jude Medical), and Mosaic (Medtronic Inc., Minneapolis, MN, US) surgical BPVs.18 In the case series by Nielsen-Kudsk (n=10), balloon-expandable THVs were only used in Mitroflow BPVs.14
Bench testing studies in vitro have assessed the durability of specific BPVs and their suitability for BVF.4,9–11 These studies have demonstrated that while the majority of commercially available BPVs can be fractured using high-pressure non-compliant balloon inflation, some surgical BPVs are unsuitable for BVF, including the Trifecta (St Jude Medical) and the Hancock II (Medtronic) BPVs.10,11 In the future, this may influence surgical BPV choice during the index procedure. One could argue that these bench tests are not a truly accurate reflection of the fracturing response of the aforementioned valves, as they have not been tested under pathophysiological conditions; that is, the BPVs tested were in pristine condition. Older valves burdened with pannus and/or calcification may respond differently to high-pressure balloon dilatation with intention to fracture.
Furthermore, the location (supra-annular versus intra-annular) of the previously implanted BPV is potentially important and requires further investigation. It has been hypothesised that there may be less risk in fracturing a BPV in the supra-annular position compared with intra-annular located BPVs.13 This warrants further study.
Balloon Type and Size
Non-compliant balloons are used (e.g. True Balloon or Atlas Gold balloons [Bard]). Typically, a size 1 mm larger than the labelled diameter of the BPV should be selected in the first instance.14 As mentioned previously, a non-compliant balloon size ≥3 mm larger than the true internal diameter of the BPV has been found to predict BVF success.8
Timing
As discussed, opinions differ as to whether BVF should be undertaken before or after implantation of the THV. This decision has generally been left to operator discretion, with no specific recommendations given, and is typically guided according to the specifics of the case.4 There are data to suggest that lower postprocedural mean gradients can be achieved when BVF is performed after VIV TAVI but, nonetheless, there remain theoretical advantages with undertaking BVF prior to TAVI implantation, which should be considered by operators. First, fracture of the BPV ring before VIV TAVI will allow confirmation that the BPV can be fractured. This may also allow for a larger-sized THV to be selected, further increasing the postprocedural effective orifice area. However, as most BPVs can be fractured, this may be unnecessary.10 Second, some have opted for BVF prior to TAVI to avoid theoretical damage to the new THV. It is argued this exposure may impact the longevity of the THV leaflets or even cause immediate damage leading to acute aortic valve regurgitation. However, it should be noted that balloon-expandable THVs are normally inflated via high-pressure semicompliant balloons at the time of implantation.
There are, however, concerns that performing BVF first may dislodge embolic material from the degenerated BPV, leaving the patient susceptible to stroke. This may have been the cause of stroke in two patients in the aforementioned large case series.8 Finally, BVF after TAVI deployment is likely to be beneficial in balloon expandable valves as discussed below.
TAVI Valve Type
Both self-expanding or balloon-expandable THVs can be used together with BVF. Bench testing studies have shown significant differences between self-expanding and balloon-expandable THV when they were implanted post BVF.9,11 It was shown that the radial force of the self-expanding THV was sufficient for complete expansion within the fractured BPV, thus requiring no additional postdilatation.9
Conversely, suboptimal results were seen when using balloon-expandable THVs (SAPIEN XT and SAPIEN 3; Edwards Lifesciences), where it was found that the delivery semicompliant balloon was not robust enough to fully expand the THV within the previously fractured BPV.9 Therefore, further postdilatation using a non-compliant balloon was required.9 To minimise this risk, fracturing the BPV post VIV TAVI was suggested.
A recent ex vivo bench study evaluated VIV TAVI and BVF with the SAPIEN 3 and ACURATE neo (Boston Scientific) THVs in 19 mm and 21 mm Mitroflow BPVs.11 It was found that a high implantation was required to enable full expansion of the upper crown of the ACURATE neo and allow optimal leaflet function. Marked underexpansion of the lower crown of this THV within the BPV was also observed. Ultimately, however, valve gradients after BVF were similar for both THVs (8.4 mmHg ACURATE neo versus 7.8 mmHg SAPIEN 3).11 The final iEOAs were 2.1 cm2 with the SAPIEN 3 and 2.2 cm2 with the ACURATE neo.11
Complications of Bioprosthetic Valve Fracture
In the aforementioned large case series, two out of 75 patients experienced postprocedural strokes (confirmed by MRI).13 Both patients fully recovered without any permanent neurological sequelae.13 There were no other direct postoperative complications observed with BVF in the three case series published to date.8,13,14 Despite these promising results, concerns remain regarding potential risks. Normally, the BPV ring offers protection against aortic annular rupture or aortic dissection during VIV TAVI, but this protection may not apply after forceful BVF. Routine CT imaging was not performed after BVF in these case series, and as such the presence of subclinical injury to the aorta could not be ruled out.
Insufficient numbers of BVF cases have been performed to confidently determine the incidence of complications, but Saxon et al. have published a review specifically highlighting complications associated with BVF.15 Documented complications include acute aortic and mitral valve regurgitation, prosthetic valve destabilisation and migration, coronary artery obstruction, balloon failure, and embolization.16,17
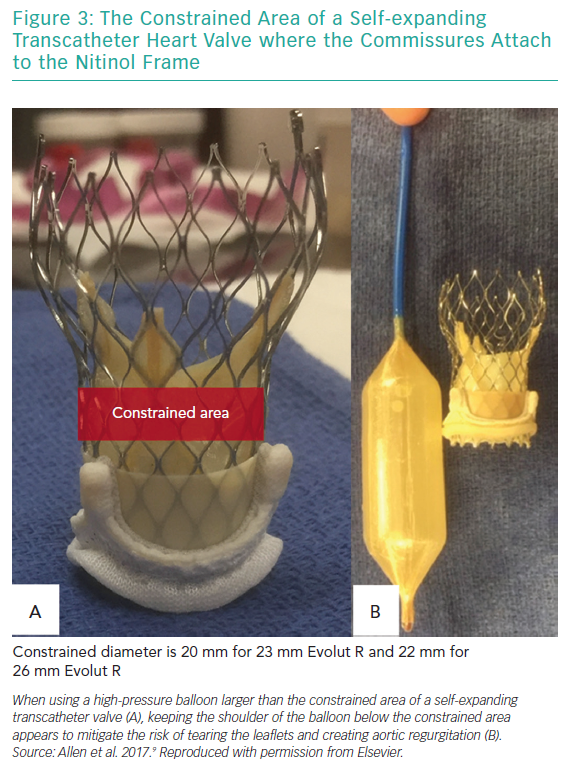
Acute valvular regurgitation is due to damage of the leaflets during balloon dilatation. The Medtronic self-expanding valve is designed with a narrowed area called the constrained area. It is within this area that the commissures attach to the nitinol frame. Therefore, inflating the balloon within the constrained region could potentially tear the leaflets. In an attempt to minimise this risk, one study proposed keeping the superior shoulder of the balloon below the constrained area during inflation (Figure 3).9
Bioprosthetic Valve Fracture in Larger Surgical Bioprostheses
Although PPM is more likely in smaller prosthetic valves, there is some evidence to suggest that underexpansion of BPVs may lead to early structural valve degeneration (perhaps because of folds in the BPV leaflets), suggesting that an upfront strategy of BVF in all degenerated surgical valves could, in theory, improve outcomes for all patients undergoing VIV TAVI.15 In the large case series reported by Allen et al., 36 of the 75 cases of successful BVF were in patients with intermediate- to large-sized BPVs (23–26 mm).8,18 This argues the potential feasibility for BVF as an adjunct to VIV TAVI in larger valves. Further clinical experience is required to provide insight into this novel technique.
Conclusion
BVF during VIV TAVI results in lower residual mean gradients, and has the potential to improve clinical outcomes among patients undergoing VIV TAVI, particularly those with small diameter surgical BPVs. Recent data suggest that it is preferable to perform BVF after, rather than before, VIV TAVI, and with a larger non-compliant balloon sized ≥3 mm, greater than the true internal diameter for better haemodynamic results. BVF appears to be safe in the limited cases series published to date but more data on larger populations of patients undergoing BVF are required.