Since the first ‘proof-of-concept’ case of transcatheter aortic valve implantation (TAVI) was reported by Cribier and colleagues in 2002, more than 200,000 patients have undergone the procedure in approximately 65 countries.1,2 Since its inception, the majority of patients undergoing TAVI have been considered inoperable or high risk for conventional surgical aortic valve replacement (SAVR) as recommended by guidelines.3 However, with increasing operator experience, technical advancements in delivery systems and transcatheter heart valves (THVs) and the systematic use of multi detector computed tomography (MDCT) for vascular access and annulus sizing, rates of important complications such as stroke, paravalvular aortic regurgitation and major vascular complications have significantly declined, resulting in lower mortality rates. This has led to a paradigm shift whereby TAVI is being increasingly performed in lower-risk patients.4–7 Therefore, surgical risk will play less of a role in patient selection in 2020 and greater emphasis will be placed on clinical and anatomical characteristics as well as patient preference. However, because all current THVs are bioprostheses, questions remain regarding the long-term durability of THVs, particularly in a younger patient population. Since the publication of the 2012 European Society of Cardiology (ESC) guidelines in which TAVI was recommended exclusively among inoperable or high-risk patients, two major randomized controlled trials – Placement of Aortic Transcatheter Valves (PARTNER) 2a and the Nordic Aortic Valve Intervention Trial (NOTION) trial – have demonstrated the safety and efficacy of TAVI among non-high-risk patients.7,8 The purpose of the present review is to provide an update on the rapidly changing field of TAVI and to provide a glimpse into the future regarding which patients will undergo SAVR and which patients will undergo TAVI in 2020.
Past
Over the last 50 years, surgical aortic valve replacement (SAVR) has become the standard-of-care for the treatment of patients with severe symptomatic aortic stenosis (AS). Nevertheless, up to one-third of patients with severe symptomatic AS were being denied treatment because they were considered too high risk to undergo the procedure, and with a global aging population this dilemma was set to be exacerbated.9 This trend was disrupted by a less-invasive, catheter-based approach to aortic valve replacement for patients with severe AS. Following the seminal work in pigs of Andersen and colleagues,10 the first-in-man TAVI procedure was performed by Cribier and colleagues in 2002.1 Since then, TAVI has evolved to become the treatment of choice for inoperable patients with symptomatic severe AS and is a viable alternative to SAVR for patients considered to be at high surgical risk. The current ESC guidelines on valvular heart disease have given a Class I indication in patients with severe symptomatic AS and a predicted survival of >1 year who are not candidates for SAVR.3 The definition of ‘inoperable’ has been problematic because widely used surgical risk scores do not capture all comorbidities that render a patient inoperable. It became increasingly clear that other factors such as frailty and anatomical factors (e.g., porcelain aorta, ‘hostile chest’, liver disease) needed to be considered. The clinical data supporting this indication have come from several European registries and the randomised PARTNER 1B study and the CoreValve (Medtronic) extremerisk registry.11–15
The current ESC guidelines also recommend that TAVI should be an alternative to SAVR in severe AS patients who are at high risk for mortality and complications after SAVR.3 The clinical evidence for this recommendation derives from several large European registries and two important randomised controlled trials comparing TAVI versus SAVR: the PARTNER 1a trial using a balloon-expandable THV and the CoreValve High-Risk study using a self-expandable THV.16,17 The 5-year echocardiographic follow-up of THVs implanted as part of the PARTNER studies have been reassuring and demonstrated haemodynamic improvements, which were equivalent to surgery at both 1 and 5 years.18 The mean gradient across the THV was stable at 10.7 mmHg and the mean aortic valve area was 1.6 cm2, with no signs of structural valve degeneration. The CoreValve US pivotal trial compared the outcomes of patients with symptomatic severe AS at high risk for surgery who underwent TAVI with a self-expanding prosthesis versus those undergoing SAVR.17 The study showed TAVI to be superior to SAVR with regards to the primary endpoint of all-cause mortality at 1 year (absolute mortality difference 4.9 %), which persisted after 2 years follow-up (absolute mortality difference 6.4 %; P=0.004).19 Furthermore, strokes were less frequent among TAVI patients after 2 years of follow-up (10.9 % versus 16.6 %, P<0.05).19 Taken together, the evidence to date suggests that TAVI is now the preferred therapy and the standard-of-care for patients with severe symptomatic AS who are not candidates for surgery. Among operable patients with severe AS who are at high surgical risk, the above-mentioned randomised trials and registries suggest that TAVI should be elevated to a Class I recommendation, as a preferred alternative to SAVR in patients who are good candidates for TAVI, especially via the transfemoral route. There are now eight commercially available TAVI systems in Europe with several that have undergone iterative changes. The main advantages for the new TAVI systems include markedly lower-profile catheter and delivery systems compared with early systems, improved operator ease-of-use, increased range of valve sizes to accommodate smaller and larger aortic annulus dimensions, retrievable and repositionable features to insure optimal valve positioning and reduced paravalvular regurgitation. Indeed, the newest generation of THVs, including the balloon-expandable SAPIEN 3 (Edwards Lifesciences) and the selfexpanding CoreValve Evolut-R (Medtronic), incorporate most of these features and have helped transform TAVI into a low-risk procedure with predictable outcomes. Comparing mortality and stroke outcomes from the earliest PARTNER randomised trials versus the most recent results from the SAPIEN 3 studies indicates a reduction in 30-day mortality from 6.3 % to 2.2 % and a reduction in strokes from 6.7 % to 2.6 %.2 Indeed, a large meta-analysis from 25 multicentre registries and 33 single-centre studies found an important reduction in strokes after TAVI over time and these findings were associated with increased operator experience and technology advancement.20 Vascular complications after TAVI, which are associated with subsequent mortality, were 32 % at 1 year in the PARTNER 1b study,14 18 % in the PARTNER 1a study with the first generation SAPIEN THV,16 17 % in PARTNER IIb with the SAPIEN XT THV,8 compared with <10 % in a recent study with SAPIEN 3.21 Because the main procedure related predictors of early and late mortality after TAVI are strokes, major vascular complications, bleeding and moderate to severe paravalvular regurgitation,22 the reduction of these complications has helped to establish TAVI as a relatively safe procedure, with mortality rates at least as low as SAVR.
Present
Whereas the first TAVI procedure performed by Cribier in 2002 was conducted under conscious sedation, location anaesthesia and without the guidance of transoesophageal echocardiography (TOE),1 procedural safety measures including general anaesthesia and intraprocedural TOE were employed thereafter in order to improve safety with large bore transcatheter delivery systems and early operator experience. However, recently the focus has shifted toward an optimised procedural approach with a simplification of the procedure.2 This strategy comprises percutaneous transfemoral vascular access, conscious sedation, reduction or elimination of intraprocedural TOE guidance, reduction or elimination of balloon pre-dilatation before valve implantation and pre-specified care plans to encourage rapid ambulation and early hospital discharge. Some high-volume centres are using this optimised approach as the standard-of-care for most TAVI patients. 23–25 However, most centres prefer a ‘hybrid’ strategy, whereby this optimised approach is used in straightforward cases and a more conventional approach in either high-risk or ‘borderline’ cases, in which TOE guidance may be beneficial (e.g., high risk for coronary occlusion or annulus rupture, valve sizing problems, chronic kidney disease etc.).2 The selective use of TOE guidance during TAVI can also be of great value in the assessment and treatment of post-implantation paravalvular aortic regurgitation and rapid diagnosis of potentially life-threatening complications such as annulus or ventricular rupture, coronary occlusion, severe aortic or mitral regurgitation (MR), bleeding or left ventricular outflow tract obstruction. However, because significant paravalvular aortic regurgitation and the aforementioned complications have gradually reduced with new TAVI devices and increasing operator experience, the optimised strategy has become prevalent in most high-volume TAVI centres. Indeed, a recent study showed that the use of an optimised transfemoral strategy reduced hospital length of stay by 40 % and total inpatient costs by US $10,000 per case compared with a standard approach using general anaesthesia and TOE guidance, without negatively affecting clinical outcomes or increasing rates of paravalvular regurgitation.26 Many TAVI centres are also developing early discharge programs to compliment the minimalist strategy. In a retrospective study of 500 high-risk or inoperable patients who underwent transfemoral TAVI, >20 % were discharged within 72 hours and there were no differences in 30-day outcomes between the two groups.27
Valve sizing has been a particularly controversial area in the recent past. After earlier attempts to measure the aortic annulus dimensions using 2D echocardiography, the current gold standard is a MDCT scan focussing on either area or perimeter measurement of the asymmetric annulus.28,29 Optimal evaluation of valvular anatomy and dimensions are crucial in order to avoid complications (e.g., annulus rupture, valve embolisation), facilitate valve positioning and prevent paravalvular regurgitation. Numerous studies have demonstrated that optimizing valve sizing with MDCT is critical in reducing the likelihood of paravalvular aortic regurgitation.28–30 Occasionally, the CT measurements fall in a grey zone between two valve sizes requiring the use of other modalities such as virtual valve implantation (e.g., using the HeartNavigator system [Philips Healthcare]), balloon sizing or 3D TOE to make the final decision regarding valve size used.31 Valve positioning is also an important consideration. Imprecise valve positioning can result in significant paravalvular aortic regurgitation, valve embolization, coronary obstruction, or conduction system abnormalities requiring permanent pacemaker insertion. However, newer generation TAVI delivery systems have dramatically improved valve positioning accuracy due to the availability of retrievable and repositionable delivery systems with newer versions of self-expanding TAVI devices and other design enhancements.
The choice of a dedicated TAVI device for the individual patient is an important consideration. It remains to be seen whether comparable clinical results can be achieved with different devices in a randomised study. This topic is of great interest as many TAVI devices differ with respect to frame geometry and composition, valve tissue properties and delivery system characteristics. Hitherto, the only THVs compared in a randomised clinical trial are the balloon expandable Edwards SAPIEN valve and the self-expanding Medtronic CoreValve bioprosthesis. The Comparison of Transcatheter Heart Valves in High Risk Patients With Severe Aortic Stenosis (CHOICE) study was a small-randomised trial in which 241 TAVI patients were randomised to receive either the SAPIEN valve or the CoreValve and be followed up at 30-days and 1-year.32,33 Although treatment with the CoreValve was associated with a higher frequency of paravalvular regurgitation and new pacemaker insertion, there were no significant differences between the two TAVR systems in 1-year clinical outcomes (death, stroke, repeat hospitalisations, vascular or bleeding events, and acute kidney injury).33 According to the clinical data available, most patients with AS who are candidates for TAVI can be treated with either an Edwards SAPIEN or Medtronic CoreValve bioprosthesis. Some anatomical circumstances may lead to favour one over another device design. For example, horizontal aortas, a heavily calcified aortic valve with protrubing calcification into the left ventricular outflow tract, bicuspid anatomy, a high risk for permanent pacemaker according to baseline ECG and patients with concerns regarding the use of rapid pacing are frequent arguments for a self expanding versus a balloon-expandable system.34 Most importantly, operator learning curves and experience with each TAVI system are major considerations. TAVI expertise requires device-specific training for correct valve sizing, accurate valve placement and avoidance of complications. Therefore, it is likely in the future that even high volume operators will restrict their TAVI device use to only a few different systems.2
The more appropriate adjunctive pharmacotherapy during and after TAVI is an important detail in clinical practice with potential high impact on clinical long-term success after procedural success. Atrial fibrillation (AF) is present in about 40 % of the elderly patient population undergoing TAVI and these patients are at high risk for both strokes and iatrogenic bleeding complications.35–36 Most studies have used a drug therapy approach consisting of intraprocedural heparin (or less commonly bivalirudin) and post-procedural dual antiplatelet therapy (aspirin and clopidogrel) for 3 or 6 months and combined with warfarin as indicated. The optimum antiplatelet and anticoagulation regimen following TAVI needs to be defined. In the phase III GALILEO trial (Global Study Comparing A Rivaroxaban-Based Antithrombotic Strategy To An Antiplatelet-Based Strategy After Transcatheter Aortic Valve Replacement To Optimize Clinical Outcomes; NCT02556203), all TAVI patients (regardless of AF status) will be randomised to receive either rivaroxaban 10 mg one daily plus aspirin 75–100 mg once daily for 3 months, followed by rivaroxaban 10 mg once daily alone, or clopidogrel 75 mg once daily plus aspirin 75–100 mg for 3 months, followed by aspirin 75–100 mg once daily alone.
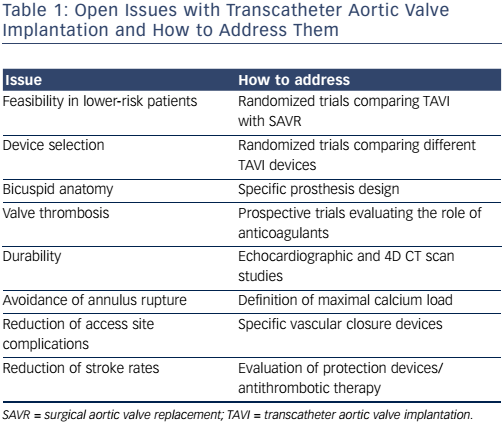
Table 1 provides a summary of the open issues with transcatheter aortic valve implantation and suggests how to address them.
Future
It has been suggested that an optimal TAVI centre should be able to achieve the following outcomes in high-risk patients with severe AS in the future: 1) all-cause mortality of approximately 2–3 % at 30 days and <10 % at 1 year; 2) significant strokes at 30 days in <2 %; 3) major vascular complications in <5 %; 4) new permanent pacemakers in <10 %; and 5) moderate or severe paravalvular aortic regurgitation in <5 %.2 These arbitrary benchmarks provide a good starting point in order to achieve optimal clinical practices. The ideal transcatheter delivery system would be less traumatic and easier to implant to further minimise the risk of vascular complications as well as paravalvular leak. The destiny of some alternative routes for the TAVI procedure (e.g., direct aortic, transapical and carotid routes) in 2020 will be limited to patients with inadequate transfemoral access. The extension of TAVI to other patient populations, including those presently being treated with SAVR and other patient subsets, is an area of great interest for the future and a number of randomised controlled trials are already underway in several of these areas. These areas include lower-risk patients, valve-in-valve for bioprosthetic valve degeneration, bicuspid aortic valve disease and treatment of severe AS and concomitant disease (e.g., AF, MR, coronary artery disease).
TAVI in Lower-risk Patients
Because TAVI was a new and unproven treatment for severe AS, the application of risk stratification to select patients for treatment was appropriate when considering the relative high frequency of periprocedural complications in the early years and the unknown durability of transcatheter bioprosthetic valves. However, after 14 years since its introduction, with over 200,000 TAVIs performed worldwide, major reductions in TAVI-related complications and reliable evidence for good medium-term valve durability, there is less justification for the imposition of strict limitations on the use of TAVI based on surgical risk stratification. In the future, as more data in lower-risk patients are accumulated, it is highly probable that TAVI will not be curtailed by risk stratification but rather will be influenced more by anatomic and clinical factors and probably patient preference. Currently, several clinical research studies have demonstrated a downward drift towards performing TAVI in lower-risk patients.4–6,37 For example, in the initial patients treated with TAVI in the Transcatheter Valve Therapy registry, the median Society of Thoracic Surgeons (STS) score for high-risk and inoperable patients was just 7 %.38 Furthermore, in the European registries and in a single-centre study, the observed 30-day mortality rate after TAVI in lower-risk patients was significantly lower than in high-risk patients.4–6,37 A recent meta-analysis of four randomised trials comparing TAVI versus SAVR (PARTNER 1A, US CoreValve, NOTION and PARTNER 2A) showed that TAVI, when compared with SAVR, was associated with a significant 13 % relative risk reduction (hazard ratio 0.87; 95 % CI [0.76–0.99]; P=0.038) for the primary outcome of all-cause mortality at 2 years.39 In subgroup analyses, TAVI showed a strong survival benefit over SAVR for patients undergoing transfemoral access and in female patients.39 Secondary outcomes of kidney injury, new-onset AF, and major bleeding favoured TAVI, while major vascular complications, incidence of permanent pacemaker implantation, and paravalvular aortic regurgitation favoured SAVR.39 Importantly, two of the included randomised trials in this meta-analysis included patients lower than high risk (PARTNER 2a and NOTION). Furthermore, the superiority of TAVI over SAVR was observed irrespective of the THV device (i.e., balloon-expandable versus self-expandable) employed across the spectrum of intermediate- and high-risk patients with similar outcome with respect to cerebrovascular accidents, stroke, and myocardial infarction. Moreover, whereas more than mild paravalvular aortic regurgitation is less frequent among SAVR patients, the post implantation THV haemodynamics (i.e., mean gradient and aortic valve area) tend to favour TAVI patients leading to a lower incidence of patient-prosthesis mismatch.39
A major limitation of randomised controlled clinical trials to date that compare TAVI with SAVR has been the use of earlier generation TAVI devices and delivery systems. A number of studies of patients with intermediate- and high-risk/inoperable AS treated with a third-generation TAVI system (SAPIEN 3 valve) have recently been published.21,40,41 The SAPIEN 3 valve is a balloon-expandable THV and consists of bovine pericardial leaflets sutured to a cobalt chromium frame. A polyethylene terephthalate skirt covers the lower portion of the frame and is designed specifically to reduce paravalvular regurgitation. The THV system is delivered through expandable 14-F (20, 23, 26 mm THV) or 16-F (29 mm THV) transfemoral delivery sheaths that expand to accommodate the device. The SAPIEN 3 THV can also be delivered via the direct transaortic or transapical routes. A recent large non-randomised registry showed excellent 30-day outcomes among intermediate-risk patients treated with the SAPIEN 3 THV.21 Among 1078 intermediate-risk patients treated with the SAPIEN 3 valve included in the multicentre registry, the rate of 30 day all-cause mortality was 1.1 %, cardiovascular mortality 0.9 %, major/disabling stroke 1.0 %, major bleeding 10.6 %, major vascular complications 6.1 % and requirement for permanent pacemaker 10.1 %.21 Transfemoral access was used in 87 %, which is important as transfemoral access enables faster recovery and shorter hospital stay as compared with nontransfemoral access.21 Furthermore, transfemoral access is associated with improved quality of life and functional status when compared with both SAVR and non-TF TAVI. A subsequent analysis compared 1-year clinical outcomes between the 1077 intermediate-risk patients (88 % via transfemoral access) from this multicentre registry with those for intermediate-risk patients treated with SAVR in the PARTNER 2a trial, using a propensity score analysis.40 The primary endpoint was the composite of death from any cause, all strokes and incidence of moderate or severe aortic regurgitation.40 For the propensity score analysis, 963 patients treated with SAPIEN 3 TAVI and 747 patients treated with SAVR were included. For the primary composite endpoint of mortality, strokes and moderate or severe aortic regurgitation, TAVI was superior (P<0.0001) to SAVR mainly driven by significant reductions in mortality and stroke, suggesting that TAVI might be the preferred treatment alternative in intermediate-risk patients.40 As compared with SAVR, all-cause mortality rates were lower at both 30 days (1.1 % versus 4.0 %) and 1 year (7.4 % versus 13 %) among TAVI patients.40 Moreover, disabling strokes were also lower at both 30 days (1.0 % versus 4.4 %) and 1 year (2.3 % versus 5.9 %).40 However, moderate or severe aortic regurgitation was still higher in the SAPIEN 3 TAVR cohort than in the surgery cohort (3.8 % versus 0.6 %) and was associated with increased late mortality.40 Mild paravalvular leak was also more prevalent among TAVI compared with SAVR patients (45 % versus 2.8 %) but was not associated with increased late mortality.
These data, combined with the encouraging 5-year valve durability findings, have increased the enthusiasm for accelerating efforts to extend the ruse of TAVI as an alternative to surgery for lower-risk patients. Indeed, the PARTNER 3 trial (NCT02675114) is a multicentre randomised controlled trial that will compare transfemoral TAVI with the SAPIEN 3 bioprosthesis with SAVR in low-risk patients (STS <4) requiring aortic valve replacement who have severe symptomatic AS. Inclusion criteria include patients ≥65 years of age with adequate iliofemoral access. The primary outcome is a composite of allcause mortality, stroke and rehospitalisation. Secondary endpoints will include new-onset AF and length of index hospitalisation. The Evolut R Low Risk trial (NCT02701283) is an open label, randomised controlled trial that will compare TAVI with the Evolut R or CoreValve transcatheter heart valves with SAVR in low-risk patients (STS <3 %). The primary endpoint is all-cause mortality or disabling stroke at 2-years. Preliminary data are expected in October 2018 for the PARTNER 3 trial and March 2018 for the Evolut R Low Risk trial. A major limitation that will affect the possibility to extend the indication of TAVI to younger patients is the high rate of permanent pacemaker implantation with certain THV prostheses.
By 2020, we predict that clinical risk assessment will be replaced by a more practical approach that relies on the knowledge of experienced TAVI operators. The multidisciplinary heart team will be of critical importance for decision-making in borderline situations, which include potentially too frail patients and patients with formal anatomical contraindications for TAVI. In individuals with favourable anatomical characteristics, patient preference will become a determinative factor.
Valve-in-valve for Bioprosthetic Valve Degeneration
The frequency of implanted surgical aortic and mitral bioprosthetic valves currently exceeds mechanical valve implantations.42 Bioprosthetic valves are mainly preferred owing to the fact that they are less thrombogenic, hence obviating the need for long-term anticoagulation. However, structural degeneration of bioprosthetic valves can result in either stenosis, regurgitation, or both, typically within 10–15 years after implantation. Therefore, the management of patients with bioprosthetic valve degeneration who are poor candidates for repeat SAVR is problematic. Since the first description of transcatheter valve-in-valve implantation in 2007,43 this procedure has become an increasingly popular strategy to treat structural bioprosthetic valve failure in high-risk and inoperable patients.44,45 On the basis of clinical registry data, the balloon expandable SAPIEN XT valve and the self-expanding CoreValve have been approved for use in high-risk patients with aortic bioprosthetic valve failure. In the largest international registry of transcatheter aortic valve-in-valve implants, early haemodynamic findings were encouraging, with 1-year survival of 83.2 %.45 Stenotic degeneration of the surgical bioprosthesis and small valve implant size were associated with worse outcomes.45 Valve-invalve therapy results in less frequent paravalvular regurgitation and new pacemakers compared with native valve TAVI but more common coronary occlusions, especially in surgical valves, in which the leaflets are externally sutured outside the stent frame. In smaller surgical valves, the self-expanding CoreValve results in less patient-prosthesis mismatch following valve-in-valve implantation because of the supraannular valve position. It is likely that in the future, transcatheter valvein-valve implantation will become the preferred therapy for surgical bioprosthesis valve degeneration in a wide spectrum of patients. Future bioprostheses will be designed to serve as a base for a potential valve-in-valve procedure in the longer-term follow-up.
Bicuspid Aortic Valve Disease
Bicuspid aortic valves have a high prevalence in younger patients with AS, but even in the elderly bicuspid valves comprise approximately 20 % of surgical cases.46 Bicuspid valves tend to have a more oval annulus shape, unequal leaflet size, and heavy eccentric calcification of the leaflets. These characteristics, together with the presence of calcified raphes, may interfere with optimal THV deployment and/or lead to suboptimal haemodynamic with increased paravalvular aortic regurgitation.47 A recent analysis of CT scans revealed that, compared with tricuspid aortic valves, the annulus was more circular and less elliptical, the annulus area and perimeter were significantly larger and there was more eccentric calcification in bicuspid valves.48 Other issues relate to the durability of bioprosthetic THVs in this patient population and the management of associated aortic pathologies, including aortic aneurysms and dissections.47 To date, only a small percentage of TAVIs are performed in bicuspid valves. A recent multicentre retrospective registry involving 12 high-volume centres (139 patients) in Europe and Canada supports the early notion that TAVI is safe among patients with bicuspid aortic valves, with a 5 % 30-day and 17.5 % 1-year mortality.49 However, post-implantation moderate to severe paravalvular aortic regurgitation was found in 28.4 % of patients, although this appeared to be mitigated somewhat by CT-based THV sizing.49 Furthermore, this registry comprised patients treated with earlier generation THVs.49 It remains to be determined whether newer generation THVs and newer technologies with THVs specific for bicuspid aortic valves may prevent paravalvular aortic regurgitation. Specific clinical trials among patients with bicuspid AS and newer generation THVs are required to help determine the most appropriate clinical indications and the most appropriate transcatheter valve design.
Treatment of Severe AS and Concomitant Disease
Elderly patients with severe AS frequently have significant cardiac comorbidities that may require additional treatment. These conditions include concomitant coronary artery disease, other valvular lesions (mitral and/or tricuspid regurgitation) and AF.
Coronary Artery Disease
Over 50 % of patients aged >70 years with severe AS also have coronary artery disease.50 The standard approach to managing patients with severe AS and CAD undergoing SAVR has been simultaneous coronary revascularisation. However, in the TAVI era, these ideas have been challenged and now selective proximal vessel, clinically-driven revascularisation is preferred rather than complete revascularisation using percutaneous coronary intervention.50,51 A number of factors must be considered in managing patients with severe AS and concomitant CAD, including the timing of percutaneous coronary intervention (before, during, or after TAVI) and the complexity of coronary lesions.50,51 Dedicated clinical trials are ongoing and should provide more definitive evidence-based guidelines.
Mitral and Tricuspid Regurgitation
MR is commonly found in patients with severe AS, and moderate or severe MR has been reported in up to 30 % of patients undergoing TAVI.52 Data from several European registries show a significant association between moderate to severe MR and 1-year mortality after TAVI.53–55 Conversely, a post-hoc analysis of the PARTNER 1A trial found that moderate to severe MR at baseline did not affect 2-year mortality among TAVI patients but did have an effect on mortality among patients undergoing SAVR.56 A recent meta-analysis by Nombela-Franco et al. revealed that 1-year mortality rates were significantly increased among TAVI patients with moderate to severe MR and that the impact of MR on mortality did not differ between self-expandable and balloonexpandable valves.57 The management strategy of patients with severe AS and concomitant moderate or severe MR depends on a number of factors including operative risk, MR severity, MR aetiology and likelihood of improvement. Key to decision making is the evaluation of MR aetiology and severity by quantitative echocardiographic methods, with the use of TOE if necessary. In selected patients with residual haemodynamically relevant MR after TAVI and continued symptoms of heart failure, a new approach is staged or simultaneous treatment with percutaneous mitral valve repair using the MitraClip system (Abbott Laboratories).58,59 Tricuspid regurgitation is also commonly found in patients with severe AS, and the combination of severe TR with right ventricular dysfunction predicts worse outcomes after TAVI.60 In the future, several methods of transcatheter TR reduction, including ‘spacers’ and plication or ring annuloplasty devices, may be combined with TAVI in selected symptomatic patients.2
Atrial Fibrillation
Pre-existing AF is present in approximately 40 % of patients with severe AS.35 New-onset AF occurs in up to 13 % after TAVI.36 AF can adversely affect cardiac physiology owing to the loss of atrioventricular synchrony and irregularity of cardiac contractility, which can result in reduced cardiac output and elevated filling pressures. AF has been associated with increased periprocedural strokes after TAVI and is associated with reduced long-term survival.35,36 The optimum anticoagulation regimen following TAVI among patients with AF needs to be defined. This can follow either a pharmacotherapy- or device-based (i.e., left atrial appendage occlusion) strategy. The latter approach in patients with AS and AF obviates the need for anticoagulation therapy and the use of combination device applications will likely become more frequent in the future.
Other Clinical Indications for TAVI
Asymptomatic Severe AS
Up to 50 % of patients with severe AS report no symptoms at the time of diagnosis and the optimal timing of intervention for these patients is uncertain and controversial.61 A recent meta-analysis found that patients with severe asymptomatic AS have a 3.5-fold higher rate of all-cause death with a watchful-waiting strategy compared with aortic valve replacement.61 Current guidelines recommend aortic valve replacement for asymptomatic severe AS in the following circumstances: 1) left ventricular ejection fraction <50 %, 2) undergoing other cardiac surgery, 3) symptoms on exercise test clearly related to AS, 4) very severe (aortic velocity >5.5 m/s) AS and low surgical risk, 5) rate of peak transvalvular velocity progression ≥0.3 m/s/year and low surgical risk, 6) increase of mean gradient with exercise by >20 mmHg and low surgical risk, 7) decreased exercise tolerance and/or abnormal systolic blood pressure response (drop or <20 mmHg rise), 8) severe left ventricular hypertrophy and 9) sustained B-type natriuretic peptide elevation.3,61,62 Given the uncertainty regarding the value of AVR in asymptomatic severe AS and the large number of affected patients, a randomised controlled trial comparing aortic valve replacement (either SAVR and/or TAVI) with conservative treatment is required.
Moderate AS and Clinical Heart Failure
Patients with moderate AS and clinical heart failure will be evaluated in a new randomised clinical trial comparing standard medical therapy versus early TAVI. The Transcatheter Aortic Valve Replacement to Unload the Left Ventricle in Patients with Advanced Heart Failure study (TAVR-UNLOAD; NCT02661451) will compare clinical outcomes among symptomatic patients (New York Heart Association class ≥2) in heart failure (left ventricular ejection fraction 20–50 %) and moderate AS undergoing TAVI with the SAPIEN 3 valve versus optimised heart failure therapy. The primary outcome will be all-cause death at 1 year and the hierarchical occurrence within one year of all-cause death, disabling stroke, hospitalisations related to heart failure and change in Kansas City Cardiomyopathy questionnaire relative to baseline.
Severe Aortic Regurgitation
Patients with severe aortic regurgitation and clinical indications for surgical repair or SAVR, but who are at high risk for surgery, have already been treated with TAVI63 and this will soon be the subject of a feasibility study in the U.S.
TAVI in Non-cardiac Surgery Sites
The 2012 ESC guidelines explicitly state that TAVI should be restricted to hospitals with both cardiology and cardiac surgery departments on-site.3 Owing to the continued evolution of TAVI to become an effective and safe treatment modality, the need for emergency cardiac surgery for complications during TAVI is currently low and about 1 %.38 A recent German multicentre registry comparing outcomes after TAVI among patients treated in centres with and without on-site cardiac surgery reported that while patients undergoing TAVI at hospitals without on-site cardiac surgery were older and at higher predicted surgical risk, major complications and in-hospital mortality were not statistically different.64 This opens the discussion about the feasibility and safety of heart-team-based TAVI at non-cardiac surgery sites.
Evolution in Technology
The current practice of TAVI has been revolutionised by the low-profile designs of current TAVI systems, dedicated user-friendly delivery systems, heart valves with proven midterm durability, precise positioning during deployment with repositioning and retrieval features and various newly developed THV mechanisms for reducing paravalvular aortic regurgitation. It is predicted that in the future TAVI systems will include even lower-profile delivery systems and ongoing refinements of THV designs to further reduce complication rates. The durability of THVs has remained a limitation in the expansion of TAVI to lower-risk patients. The 5-year follow-up data from PARTNER is reassuring in that there were no episodes of structural valve failure and mean gradient and effective orifice areas remained stable.18 However, longer follow-up data on THVs is required to determine whether they are equivalent to the most durable surgical bioprosthetic valves. With the possibility of transcatheter valvein-valve treatment to extend the duration of THVs it is possible that the uncertainty regarding long-term durability may become less concerning.
Stroke and TAVI
Stroke is among the most feared complications of TAVI because of the burden of morbidity and mortality. With increased operator experience and newer generation devices, overall rates of cerebrovascular incidents have declined to rates ranging between 1.5 % and 6 %. Disabling stroke rates amount to 1–3 % at 30 days. Nevertheless, systematic neurologic and neuroimaging follow-up in TAVI and SAVR patients has just started to become of crucial interest, and silent neurologic deficits and cerebral ischaemia is reported in up to 15–28 % of patients. Even though not always clinically apparent, these cerebral lesions have been linked in non-TAVI patient populations to cognitive decline, an increased risk of subsequent dementia, and a >3-fold increased risk of future stroke.65–67 Several cerebral protection devices, such as the Embrella Embolic Deflector system, the Triguard HDH Embolic Deflection device or the Claret device have been developed to capture freed embolic material during TAVI procedures. Neuroimaging studies have shown improvements suggesting increased cerebral perfusion with cerebral protection in early randomised clinical trials.68,69 However, it remains unclear whether these devices should be used systematically versus selectively in high-risk patients undergoing TAVI. In the SENTINEL IDE trial (trial registration number NCT02214277), 356 patients have been randomised to embolic protection or no embolic protection, with the aim of evaluating the reduction in median total new lesion volume between the test and control cohorts, assessed using MRI studied at 2–7 days after TAVI. Further data from ongoing clinical trials should shed light on this issue. A number of other accessory devices have been developed and include dedicated preshaped guidewires, improved temporary pacemaker technologies, novel percutaneous closure devices and new valvuloplasty systems.2
Conclusion
In order to predict the future, one must create it. Over the past 14 years, TAVI has evolved to become a viable alternative to SAVR for patients with severe AS with high or excessive risk. Rapid advances in technology have resulted in a simplified procedure and reduced complications. In the future, THV durability and expansion of clinical indications (e.g., asymptomatic severe AS, moderate AS and heart failure, severe aortic regurgitation) will be important issues. By 2020, it is highly probable, given the aforementioned improvements in complication rates and clinical outcomes, that the majority of patients with AS (and in the longer term, the majority of patients with aortic valve disease) will be treated with a transcatheter based bioprosthesis system.70 The use of these systems will be expanded in parallel to treat non-aortic valvular heart diseases as already demonstrated in ‘offlabel’ cases.71 Dedicated transcatheter devices for the different valves are meanwhile in development.