From the early days of percutaneous coronary intervention (PCI) it became apparent that the presence of severe coronary calcification was a predictor of worse clinical outcomes. In the era of plain old balloon angioplasty, severe coronary calcification was associated with an increased risk of coronary dissection and procedural failure, while in the bare-metal stent era, it was associated with a higher incidence of in-stent restenosis and target lesions revascularisations (TLRs).1,2 The advent of drug-eluting stents (DES) changed the landscape of coronary intervention through the reduced risk of restenosis and TLR, thereby allowing the interventional treatment of complex lesions and high-risk patients. However, a recent patient-level pooled analysis from seven contemporary stent trials revealed that patients with severely calcified lesions still have worse clinical outcomes compared with those without severe coronary calcification.3 Patients with severe lesion calcification were less likely to have undergone complete revascularisation, resulting in a higher residual Syntax score, which is a powerful determinant of prognosis.4
Although rotational atherectomy was expected to be one of the solutions, the prospective, randomised ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial did not demonstrate any better clinical outcomes.5,6 The latest ACCF/AHA/SCAI and ESC/EACTS PCI guidelines and European expert consensus on rotational atherectomy state that rotational atherectomy has a limited role in facilitating the dilation or stenting of lesions that cannot be crossed or expanded with PCI.7–12
Rotational atherectomy should not be performed routinely for de novo lesions or in-stent restenosis.13
Recently, a newly developed atherectomy device, a Diamondback 360® Coronary Orbital Atherectomy System (OAS) (Cardiovascular Systems Inc.) has been approved by the US Food and Drug Administration (FDA) based on the results of the Evaluate the Safety and Efficacy of OAS in Treating Severely Calcified Coronary Lesions (ORBIT) II trial14 and is a new treatment option for severely calcified coronary lesions. The purpose of this review is to provide insights for procedural considerations and patient selection from the currently available publications assessing the OAS.
Diamondback 360® Coronary Orbital Atherectomy System
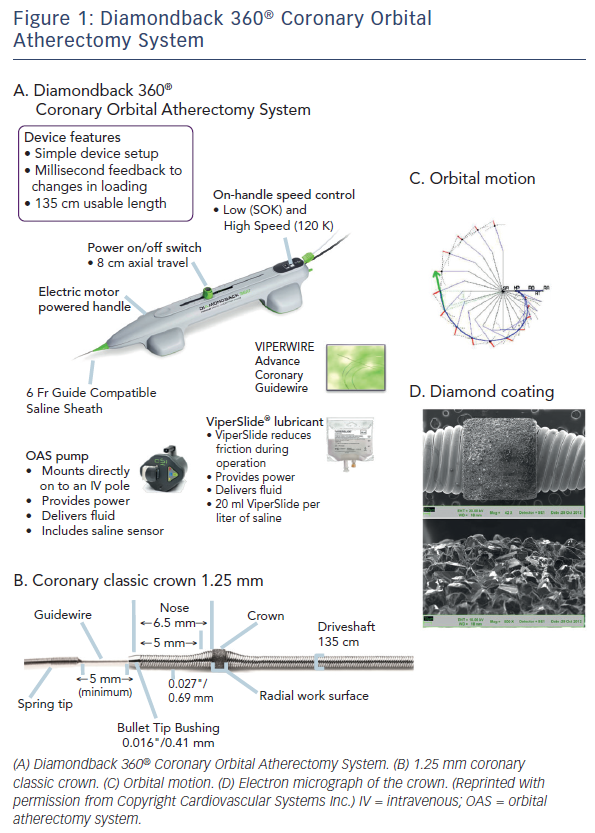
The Diamondback 360® Coronary OAS is the device to facilitate stent delivery in patients who are acceptable candidates for PCI due to de novo, severely calcified coronary artery lesions (see Figure 1). The Diamondback 360® Coronary OAS is the device to facilitate stent delivery in patients who are acceptable candidates for PCI due to de novo, severely calcified coronary artery lesions. In October 2013, Cardiovascular Systems Inc. received FDA approval for the use of the Diamondback Coronary OAS in the US, whereas it has yet to receive CE Mark in Europe. Only one size of crown (1.25 mm) is required for the coronary OAS. ViperWire Advance® coronary guide wire (335 cm) is to be used exclusively with the Diamondback 360® Coronary OAS to enable optimal orbital path and efficient differential sanding.
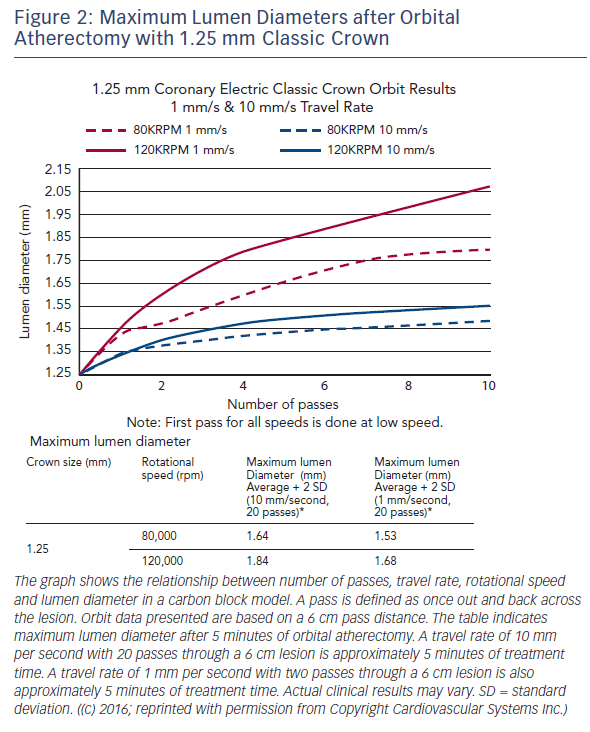
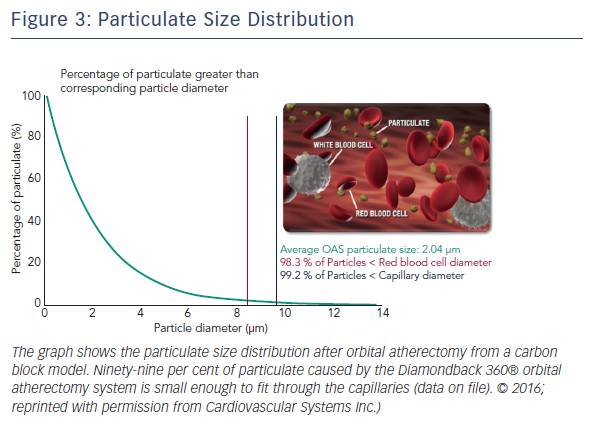
The Diamondback 360® Coronary OAS uses a differential sanding mechanism of action to reduce plaque while potentially minimising damage to the medial layer of the vessel. Softer tissue flexes away from the crown while fibrotic tissue or arterial calcium is engaged and treated facilitating stent deployment. A drive shaft with an eccentrically mounted diamond-coated crown provides proximal and distal sanding to reduce occlusive material and restore luminal patency.15 The crown’s orbital diameter expands radially via centrifugal force according to the following formula: F = mv2/R (F = centrifugal force; m = mass of the crown; v = velocity [device rotational speed]; R = radius of rotation) (see Figure 1). Operators can control the speed of rotation with the knowledge that a higher speed will create a larger sanding diameter by increasing lateral pressure (see Figure 2). Based on carbon block testing, the average particle size created by OAS is 2.04 μm; 98.3 % of particles are smaller than red blood cell diameter; and 99.2 % of particles are smaller capillary diameter (see Figure 3). In the US, a physician training and certification programme based on physician atherectomy experience is required to use OAS. All physicians must complete an online training module and complete six clinical proctored cases. Based on their experience level they may also need to attend a preceptorship course.
Procedural Consideration for Coronary Orbital Atherectomy System in Comparison with Rotational Atherectomy
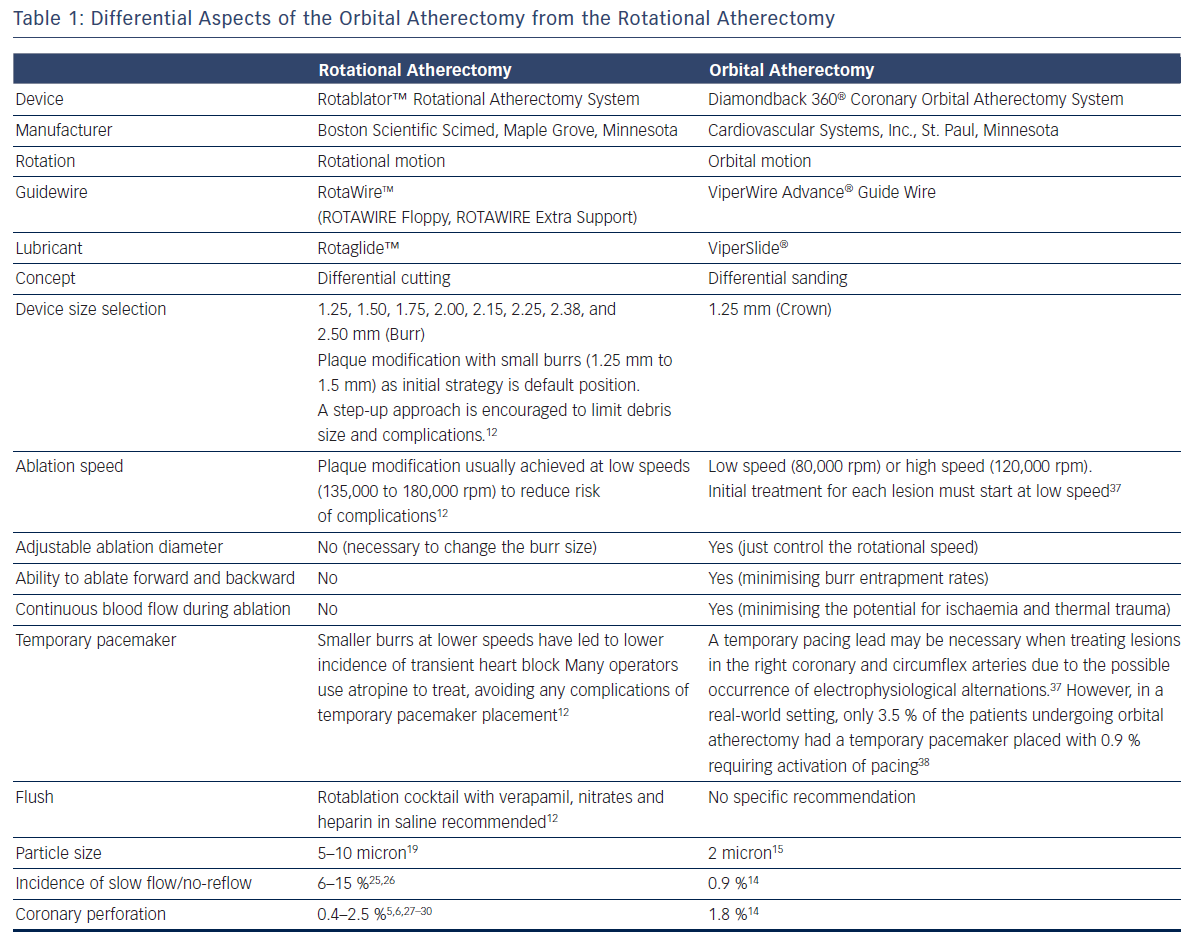
Basic procedural consideration for orbital atherectomy can be similar to that of rotational atherectomy.12,16,17 Differential aspects between orbital atherectomy and rotational atherectomy are summarised in Table 1.
One advantage of the Diamondback 360 Coronary OAS is the ease of use. The electric handle allows the user to simply plug in the device, and the only portion of the Diamondback 360 Coronary OAS that is not in the operating field is the saline infusion pump.18 The orbital path of the device around the periphery of the lumen allows the crown to attack the plaque, in contrast with the burr of a rotational device, which remains in one place. In both rotational atherectomy and orbital atherectomy, a healthy, compliant tissue should flex away, whereas fibrotic calcific lesions would generate an opposing force, allowing differential cutting and differential sanding, respectively. The OAS uses a principle of off-axis centrifugal force, with the orbital motion diameter being proportional to the applied speed. Operators can adjust the ablation diameter by controlling the rotational speed without changing the device crown size (see Figure 2). The unique crown shape and diamond coating enable ablation of severely calcified lesions forward and backward, minimising burr entrapment rates. The device allows constant blood and saline flow and particulate flushing during orbit, which facilitates cooling, minimising the potential for ischemia and thermal trauma, which can be a potential cause of restenosis. In comparison, rotational atherectomy uses a concentric burr that does not allow blood and micro-debris to flow past the burr. The average particle size created by rotational atherectomy is 5–10 μm,19 while the average particle size by orbital atherectomy is approximately 2 μm,15 resulting in its lower incidence of slow flow or no reflow than that of rotational atherectomy.
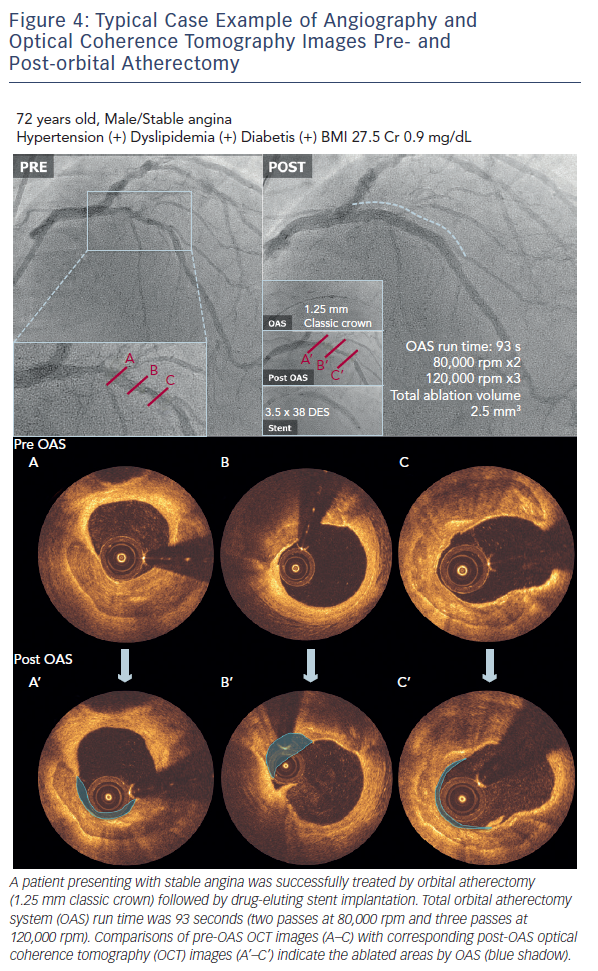
A case example treated with the OAS is demonstrated in Figure 4. OCT images pre- and post-orbital atherectomy show the appearance of ablated plaque. The ‘guidewire bias’ (intraluminal [eccentric] position of the supporting wire) seems important for the effective ablation not only during rotational atherectomy but also during orbital atherectomy. In addition, imaging evaluation by intravascular ultrasound (IVUS) or OCT after the orbital atherectomy might be recommended for sufficient lesion preparation,20,21 since the ablation diameter largely varies depending on the run time, number of passes and rotational speed (see Figure 2). Without imaging assessment, the efficacy of orbital atherectomy could highly depend on the experience of operators.
Clinical Evaluation for Coronary Orbital Atherectomy System
We conducted a literature search to identify all published coronary OAS studies and Summarized their results in Table 2. Two landmark trials (ORBIT I and ORBIT II) from India and the US have demonstrated the clinical safety and efficacy of the OAS, resulting in the approval by FDA in 2013. No clinical data in a direct comparison study between orbital atherectomy and rotational atherectomy is available so far.
The first-in-man assessment of the coronary OAS to treat de novo calcified coronary lesions was performed in the ORBIT I clinical trial.22 Fifty patients with de novo calcified coronary lesions were enrolled in this non-randomised, multi-centre trial in India. Procedural success, defined as ≤20 % residual stenosis after stent placement, was achieved in 94 % of patients. There was no incidence of slow flow or distal embolisation. In contrast to previous PCI trials with rotational atherectomy, ORBIT I reported low rates of major adverse cardiac events (MACE) (6 % at 30 days and 8 % at 6 months).22 Long-term follow-up was collected on a single-centre subset of ORBIT I subjects enrolled at CIMS Hospital, India (n=33). Of the 33 subjects, the observed MACE rate at 2 years was 15 % (5/33), 3 years was 18 % (6/33) and 5 years 21 % (7/33).23,24
The ORBIT II trial was a prospective, single-arm multicentre, non-blinded clinical trial that enrolled 443 consecutive patients with severely calcified coronary lesions at 49 US sites from 25 May 2010 to 26 November 2012.14 The Diamondback 360® Coronary OAS was used to prepare severely calcified lesions for stent placement. The primary safety endpoint was 89.6 % freedom from 30-day MACE compared with the performance goal of 83 %. The primary efficacy endpoint (residual stenosis <50 % post-stent without in-hospital MACE) was 88.9 % compared with the performance goal of 82 %. Stent delivery was performed successfully in 97.7 % of cases with <50 % residual stenosis in 98.6 % of subjects. Low rates of in-hospital Q-wave MI (0.7 %), cardiac death (0.2 %) and target vessel revascularisation (0.7 %) were reported. The incidence of slow flow or no reflow in the rotational atherectomy has been reported to be 6 % to 15 %,25,26 whereas in the ORBIT II trial the rate of persistent slow flow/no reflow for orbital atherectomy were notably very low, occurring in 0.9 % of patients.14 Perforations occurred in 1.8 % of patients compared with 0.4 % to 2.5 % in the several rotational atherectomy studies reporting on this complication.5,6,27–30 The ORBIT II perforation rate is within the previously reported range. The ORBIT II trial met both the primary safety and efficacy endpoints by significant margins and not only helped facilitate stent delivery, but also improved both acute and 30-day clinical outcomes compared with historical controls in this difficult-to-treat patient population.
The comparison study by Kini et al. assessed the mechanistic difference of impact by rotational atherectomy and orbital atherectomy with OCT.31 Although the number of the study population was limited, precise imaging analyses revealed that tissue modification with deep dissections in around a third of lesions after rotational atherectomy and orbital atherectomy; however, post-orbital atherectomy dissections were significantly deeper than post-rotational atherectomy (1.14 versus 0.82 mm; P=0.048). Stents after orbital atherectomy were associated with a significantly lower per cent of stent strut malapposition than those after rotational atherectomy (4.36 versus 8.02 %; P=0.038).
In comparison with rotational atherectomy, more significant modification of heavily calcified plaques by the orbital atherectomy led to better stent expansion and apposition, which might result in a lower MACE rate in the previous two landmark studies.
Patient Selection for Coronary Orbital Atherectomy System
The indication of rotational atherectomy according to the expert consensus and guidelines is for calcified lesions, which, in the absence of plaque modification, confer an increased likelihood of procedural failure, stent under expansion, restenosis and major complications.9,10,12,32 Although routine use of rotational atherectomy did not improve outcomes after DES implantation,5,6 such a device might technically be required in cases of tight and calcified lesions, to allow subsequent passage of balloons and stents. In most cases, the simple passage of a single burr is sufficient to smoothen the vessel lumen, or to disrupt the continuity of intravascular calcium rings, to enable subsequent balloon dilatation and stent implantation.
Orbital atherectomy is indicated for severe calcium of coronary de novo lesions. In addition, since the OAS could simplify the procedure with its small crown size and adjustable ablation diameter with the rotational speed control, the procedural simplification could extend the clinical application of the orbital atherectomy device to several potential situations as follows.
Multi-vessel Disease With Severely Calcified Lesions
Patients with multi-vessel disease and severely calcified lesions are less likely to have undergone complete revascularisation,3 resulting in a higher residual Syntax score which is a powerful determinant of prognosis.4 Both rotational atherectomy and orbital atherectomy can facilitate the device delivery and complete revascularisation, which could help achieve a lower residual Syntax score. So far there is not robust evidence that the decalcification strategy with rotational atherectomy and extensive metallic stent implantation could improve clinical outcomes.5,33 This should be investigated and proved with use of orbital atherectomy.
Lesion Preparation for Implantation of Bioresorbable Scaffolds
Bioresorbable scaffolds appear to overcome the limitations of the permanent metallic stents, but technically require more extensive lesion preparation, especially in calcified lesions due to its limited mechanical strength. There is resurgence in the use of atherectomy for the purpose of optimal lesion preparation among patients undergoing implantation of bioresorbable scaffolds.34
Ostial lesions, Unprotected Left Main Disease, Chronic Total Occlusions and Stent Ablation
As described in the European expert consensus on rotational atherectomy,12 there might still be angiographic settings where a more extensive ablation is desirable, i.e., ostial lesions, unprotected left main disease, chronic total occlusions and stent ablation. Ostial lesions, unprotected left main disease and chronic total occlusions have not been studied in a clinical trial with orbital atherectomy, but crossable lesions might be safely treated with a small crown size and adjustable ablation diameter with the rotational speed control. Stent ablation is contraindicated for orbital atherectomy. Whether orbital atherectomy would improve the outcomes of these lesion settings needs to be proved by future clinical studies.
Cost-effectiveness
Chamber et al. reported that the coronary OAS device offered a projected cost savings compared with the pooled population of the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI)/Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trials, as high as $4,913 (€4,341), which was enough to offset the cost of the device, while still providing additional savings to the hospital.35,36 Specifically, reduced procedural complications, length of stay and readmission rate contribute to the cost-effectiveness. The economic study based on ORBIT II concluded that even in a low-value scenario, OAS offers a cost per life-year gained or incremental costeffectiveness ratio of $11,895 (€10,511). In the US, treatments are generally considered high value when they cost less than $50,000 (€44,181) per life-year gained, and the OAS result was well below that threshold.
Conclusion
The recent results of OAS trials appear to be encouraging; however, further trials in a randomised fashion are still required to conclude the true value of the OAS. The necessity for sufficient lesion preparation before implantation of bioresorbable scaffolds serves as important driving forces in performing a randomised study to compare the long-term outcomes of the two atherectomy devices and balloon angioplasty in patients with severely calcified lesions.