During the past decade, transcatheter aortic valve implantation (TAVI) has revolutionised the interventional treatment of aortic stenosis (AS).1 It has rapidly evolved from a treatment used on a compassionate basis for inoperable patients to being the standard of care in high-risk AS.2,3 In that cohort it has proven to be non-inferior to surgical aortic valve replacement (SAVR) in terms of mortality, and superior to optimal medical treatment (OMT) in terms of mortality and rehospitalisations.4,5 Evidence and indications are now moving towards its suitability for intermediate and low-risk profiles.
Randomised trials such as Placement of Aortic Transcatheter Valves (PARTNER 2) and Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients (SURTAVI) have shown that TAVI is a safe and effective treatment option for people with intermediate risk; being non-inferior to SAVR overall and superior to surgery when it is performed using the transfemoral approach.6,7 The randomised controlled Nordic Aortic Valve Intervention (NOTION) trial, which included intermediate and low-risk patients, has also confirmed the safety and efficacy in a low-risk setting.8 It is important to clarify that low-risk patients are not necessarily younger patients (the mean age in all the mentioned studies was about 80 years). This leaves unanswered questions about valve durability; as far as we know the incidence of structural valve deterioration after 5 years is very low, but information beyond this time is scarce.9–12
The use of TAVI is continuing to evolve worldwide. Transcatheter heart valves (THV) are being used for valve-in-valve treatment in failing bio-prostheses, treatment of bicuspid aortic valves in younger patients with complex anatomical features and for native pure aortic regurgitation (NPAR). This is currently an off-label indication as it poses multiple challenges with variable and unpredictable immediate and long-term results.13–15 The aim of this review is to describe: the main differences between AS and NPAR; its impact on procedure complexity; the THVs available for NPAR treatment and current evidence regarding success and short-term results.
Native Aortic Valve Regurgitation
From a pathophysiological point of view, severe AS is characterised by pressure overload with consequent concentric hypertrophy and afterload mismatch.16 In most cases, after TAVI is performed and this mismatch corrected, left ventricular ejection fraction increases and there is regression of left ventricular (LV) hypertrophy.17 This explains why patients have the clear benefits of quality of life and life expectancy after TAVI.
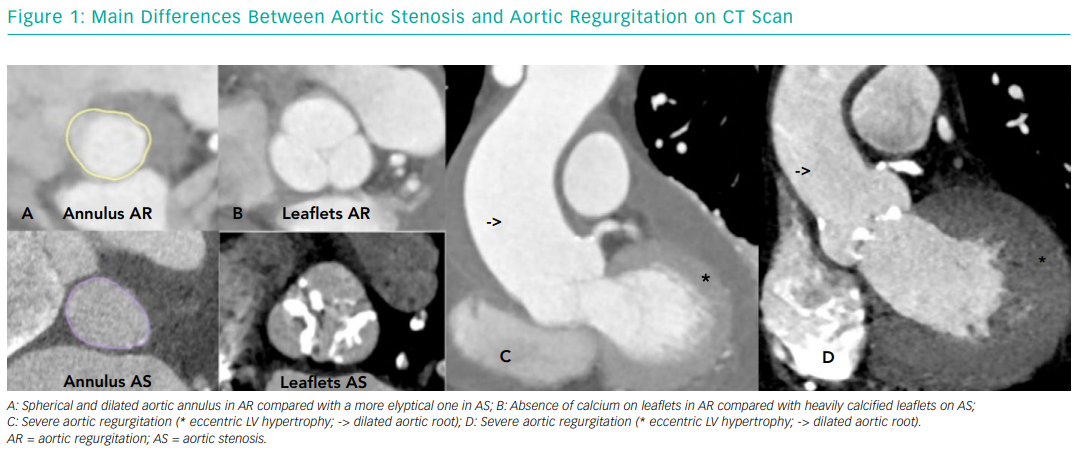
On the other hand, severe NPAR is characterised by volume overload and eccentric hypertrophy (increased ventricular volume with little increase in wall thickness and increased LV wall stress) associated with LV cavity structural modifications and progressive LV dysfunction.18 These structural modifications are due to cardiomyocyte enlargement triggered by multiple growth factors that modulate cardiac output by means of the Frank-Starling mechanism. Once the Frank-Starling mechanism is lost, LV function is irreversibly impaired.19,20 From an anatomic point of view, degenerative AS results from progressive calcification of the aortic valve leaflets and annulus, while NPAR is usually the result of leaflet degeneration or incompetence, aortic root dilatation with aortic annulus enlargement, or both. These anatomical differences pose particular challenges for TAVI, which we will discuss later. Figure 1 depicts the main anatomical differences between AS and AR.
Current Management of Aortic Regurgitation
Prevalence of AR increases with age and it affects about 13% of patients with isolated, native left-sided valvular heart disease.21 Symptoms related to AR tend to appear late in the history of the disease, once LV dilatation and systolic dysfunction have set in. Patients with severe AR and an ejection fraction <30% have an annual mortality risk of 20%, but unfortunately only 5% of these patients are given SAVR according to data from the Euro Heart Survey on Valvular Heart Disease.21
According to current European and US guidelines, patients with symptomatic moderate/severe AR and decreased LV systolic function (<50%) or severe LV dilatation (LV end-systolic diameter >50 mm; LV end-diastolic diameter >65–70 mm; LV end-systolic volume index >45 ml/m2) should be considered for SAVR.2,3 Nonetheless, there is a high-risk subgroup who are inoperable and who could be considered for TAVI, taking into account the multiple procedural challenges and the fact that it still is an off-label indication.22 To date, the standard of care for severe NPAR is SAVR with TAVI emerging as an option for high-risk or inoperable patients.
Technical Challenges During TAVI for NPAR
The main challenge that interventionists face during TAVI for NPAR is the absence of annular and leaflet calcification, which is necessary for device anchoring and stabilisation during deployment. The lack of calcium, the increased stroke volume secondary to severe AR and the presence of aortic root dilatation makes device positioning and deployment very difficult and there is a predisposition to embolisation or malposition of the prosthesis with subsequent moderate to severe post-procedural AR (associated with worst clinical outcomes).23 Valve migration can occur to the aorta or deep into the LV up to several hours after implantation.24 Valve oversizing has been proposed to reduce the risk of valve migration. Published data recommend a 15–20% oversize when selecting the THV size with the caution not to oversize beyond 20% due to the risk of annular rupture and conduction system abnormalities.25,26
THV Devices Available for NPAR
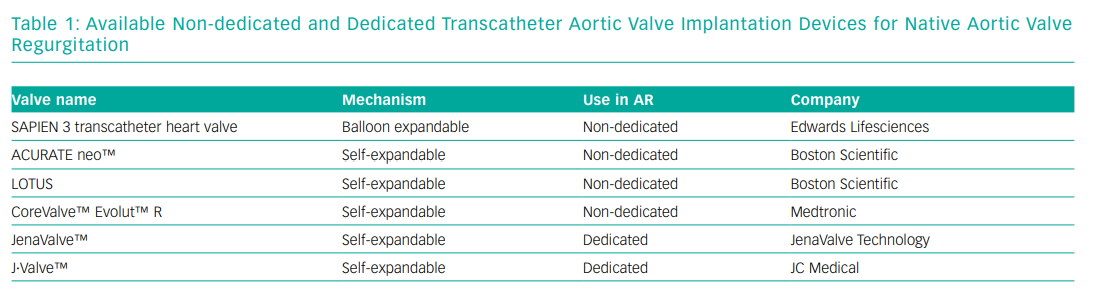
Second generation THVs that have been used for TAVI for NPAR can be divided into non-dedicated devices: CoreValve Evolut R (Medtronic), Sapien 3 (Edwards Lifesciences), Lotus valve (not commercially available at time of writing) and ACURATE neo (Boston Scientific) and dedicated devices: JenaValve (JenaValve) and the J.Valve (JC Medical).27 Non-dedicated devices are widely used for TAVI for AS (their mechanisms are dependent on annulus and leaflet calcification for fixation), while the dedicated devices have been developed to be implanted in non-calcified valves anchoring in the aortic annulus and clipping the native valve leaflets for stability.28
Self-expandable THVs have been the preferred non-dedicated devices used for TAVI for NPAR with CoreValve being the most widely studied. Self-expandable THVs can be recaptured and repositioned which theoretically make the prosthesis behave in a more predictable manner.29,30 Experience with ACURATE neo (transfemoral) is limited to successful case reports and there is a small series of eight patients treated with ACURATE TA (transapical) that showed good results based on its hourglass design, stabilisation arches and upper crown that ensure coaxial alignment and device stability during deployment.31,32 The first successful transfemoral implantation of a Lotus valve in pure NPAR was reported in 2016 and the authors warrant its use based on its repositionability and retrievability.33
Sapien 3 valve (balloon expandable) implantation has been shown to be feasible for NPAR in three cases reported in 2016. Deployment position was more ventricular than that recommended for AS and the annulus oversizing ratio was >15% using from 3 to 10 mm extra volume according to the LV outflow tract dimensions.34,35
The JenaValve, a self–expanding, 32Fr transapical valve with three integrated locators was the first dedicated device to get the CE mark for NPAR based on its anatomically correct positioning in the native cusps and clipping of the THV onto the native leaflets.36,37 Since June 2016, the transapical system is no longer available but development of a new generation transfemoral system is underway and has been used successfully for NPAR in a first-in-human case report in 2017.38 The Longterm Safety and Performance of the JenaValve (JUPITER) registry showed a procedural success rate of 96.7% with 0% incidence of valve malposition and moderate to severe post-procedural AR.39
Another NPAR-dedicated second generation TAVI device is the J-Valve, which has a unique system composed of three U-shape graspers that facilitate intuitive self-positioning implantation providing axial and radial fixation by embracing the native valve leaflets. A successful first-in-human implantation was reported in 2015 but currently, the device is only available in Asia.40 Table 1 shows the different THVs available for NPAR.
TAVI for NPAR: Evidence on Early Generation Devices
The first use of TAVI for NPAR was reported by Roy et al. in 2013 and included the retrospective analysis of 43 patients at 14 centres who had TAVI for severe inoperable NPAR. All cases used CoreValve prosthesis and as part of the procedure protocol two pigtail catheters in different sinuses of Valsalva were used to guide THV delivery under rapid pacing. Results included a 97.7% success rate (according to protocol and not VARC-2 guidelines), in 18.6% of the cases a second valve was required during the index procedure for residual AR (all of which had absent valve calcification) and the one-year all-cause mortality was 21.4%.41 Multiple studies followed using early generation devices (CoreValve being the most used followed by Sapien/Sapien XT, JenaValve, Direct flow and ACURATE TA), and in 2016 Franzone et al. published a meta-analysis of 13 studies with a total of 237 severe inoperable NPAR patients without AS treated with TAVI.42–44 In 80% of the cases a self-expandable valve was used and less than 25% of the cases were treated with devices approved for AR. Device success ranged from 77% to 100% with a 7% incidence of second valve implant due to either device migration or severe post-procedural AR. The primary endpoint of all-cause mortality at 30 days ranged from 0 to 30% with a summary estimate rate of 7%. Moderate to severe post-procedural AR was reported in up to 88% of patients with a summary estimate rate of 9%.45 The JenaValve subgroup had a 0% incidence of moderate to severe post-procedural AR. Given the heterogeneity of the groups and procedural aspects, no solid conclusions in terms of safety and efficacy can be drawn from these initial experiences but all of them showed that TAVI for NPAR is complex, with success rates below those reported for AS and a high incidence of valve malposition and moderate to severe post-procedural AR.
TAVI for NPAR: Evidence on New Generation Devices
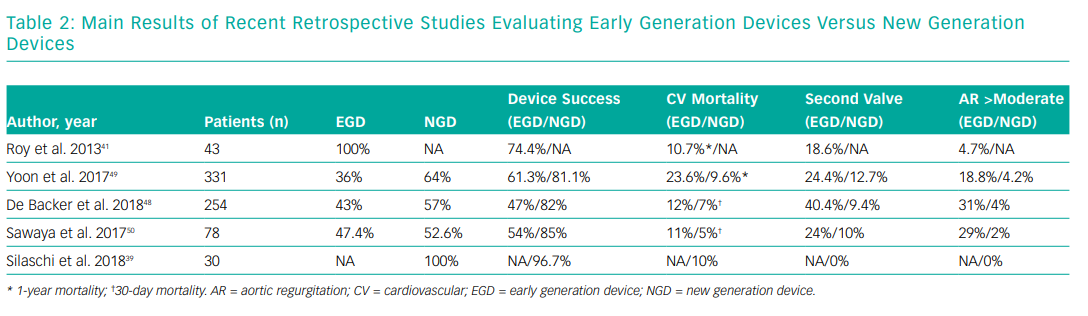
New generation devices (NGDs) such as CoreValve Evolut R, ACURATE neo, Lotus valve and Sapien 3 have features that distinguish them from their predecessors. Characteristics such as retrievability and repositioning in the case of the self-expandable valves and the adaptive seal or skirt found in Sapien 3 and Lotus valve offer a more controlled and predictable TAVI procedure.46,47 Three recent retrospective studies have analysed the use of new generation TAVI devices for NPAR and compared their results with early generation devices.
De Backer et al. reported the early safety and clinical efficacy of TAVI for NPAR in 254 patients from 46 centres with an EGD/NGD proportion of 43% and 57%, respectively. Overall device success according to Valve Academic Research Consortium (VARC-2) criteria was 67%, being higher with NGD (82% versus 47% when compared with EGD).9 NGD use was associated with less valve malpositioning and less moderate to severe post-procedural AR. Cardiovascular mortality was also lower with NGD. As part of the study they focused on THV CT-scan sizing and found a significant increase on the incidence of device embolisation with relative THV under or oversizing when compared with neutral sizing. The authors found no causal explanation for this phenomenon other than valve design and absence of calcification.48
Yoon et al. reported 331 severe NPAR patients from 40 centres (36% EGD and 64% NGD). Primary endpoint was all-cause and cardiovascular mortality at one year. Overall device success was 74.3% and again, second valve implantation, moderate to severe post-procedural AR and cardiovascular mortality were significantly lower with NGD (12.7% versus 24.4%; 4.2% versus 18.8% and 9.6% versus 23.6%, respectively) when compared with EGD. They also found that the absence of calcium or the presence of mild calcification was associated with less frequent device success with EGD but not with NGD. A larger annulus (>25.2mm) was associated with less frequent device success either with EGD or NGD. Finally, they showed that a higher degree of perimeter oversizing index (>15%) was associated with less frequent moderate to severe AR.49
Sawaya et al. performed a retrospective analysis of 78 patients with severe NPAR treated with TAVI. The majority of cases were done under general anaesthesia via transfemoral access with CoreValve; given its radial force at both the annular level and ascending aorta and also because it could be significantly oversized without risk of annular rupture. Results were consistent with those that we have previously described. NGD showed a lower incidence of valve malposition, a lower degree of AR and cardiovascular mortality versus EGD. They also found that a BMI <20kg/m2, Society of Thoracic Surgeons score >8%, major vascular complication or new left bundle branch block, and more than moderate AR were independent predictors of mortality and New York Heart Association III–IV at 30 days after TAVI for NPAR.50 All of these findings were confirmed in a recent meta-analysis.51 Table 2 summarises the main results of these three studies.
Interventional Tips and Tricks for TAVI for NPAR
The following recommendations are made based on personal experience and information gathered from published cases. The first and one of the most important steps before TAVI for NPAR is a pre-procedural CT assessment with focus on the annulus area, sinuses of Valsalva and aortic root diameters and device size selection keeping in mind that a 15–20% oversizing index is recommended in this setting (oversizing index formula: [(device nominal perimeter/area) / (annulus/perimeter area measured by CT) – 1] x 100.52 The procedure should be carried out preferably under general anaesthesia, as it can be lengthy and complicated. Transoesophageal echocardiogram can be used to help with valve positioning but more importantly to accurately evaluate the degree of post-procedural AR. Given the absence of calcification and fluoroscopic landmarks many operators use two pigtail catheters in different sinuses of Valsalva, or CT fusion-guided imaging for valve deployment. Balloon predilatation should not be performed unless it is used to measure the annulus when there is no available CT. Rapid pacing is mandatory for balloon expandable valves and it can also be used with self-expandable valves to reduce the stroke volume, helping to stabilise the aortic annulus and limit THV motion by reducing the regurgitant jet. While deploying the valve, always pay close attention to the haemodynamic profile, particularly to the waveform, the dicrotic notch and the aortic diastolic pressure.53 Whenever using a balloon-expandable valve, keep in mind that variable amounts of extra volume should be added to avoid valve embolisation. Finally, based on the evidence we have presented, the use of NGD should be mandatory in TAVI for NPAR.
Conclusion
Patients with NPAR who are candidates for TAVI tend to be in a poorer clinical condition than many contemporary AS patients, due to LV dilatation and dysfunction. These facts alongside the technical difficulties met during the procedure and the lack of transfemoral dedicated devices make TAVI for NPAR an ‘off-label’ treatment. Even though better results are achieved with NGD in terms of lower rates of valve malposition/second valve insertion during index procedure and lower incidence of moderate to severe post-procedural AR, clinical results are far from those achieved with TAVI for AS. To date there are no randomised clinical trials and all the evidence we have comes from retrospective studies with heterogeneous populations and no standardised TAVI protocol for NPAR.
New dedicated devices are being designed and those available are evolving to transfemoral as we continue to gain experience of using non-dedicated devices for this patient group. Nonetheless, TAVI for NPAR has to be considered the treatment of choice for inoperable severe AR patients because it offers a better prognosis than optimal medical treatment. TAVI has been established for inoperable or high-risk patients, but we need to improve. TAVI is not yet the standard of care for NPAR, but it is likely to be established as such in time.