Acute coronary syndromes (ACS) encompass a wide spectrum of clinical presentations that range from ST-elevation MI (STEMI) to non-ST-elevation MI and unstable angina. These conditions are life threatening and remain a source of high morbidity and mortality. Unfortunately, despite major accomplishments worldwide in timely reperfusion with percutaneous coronary intervention (PCI), an important residual risk of future cardiovascular events and mortality persists.1
Numerous methods have been proposed to provide individualised management in patients with ACS. In the past 20 years, there has been an exponential increase in the application of epicardial functional indices (e.g. fractional flow reserve [FFR]) and microvascular indices (e.g. index of microcirculatory resistance [IMR]). The initial proof-of-concept validation for these indices was performed in patients with chronic coronary syndromes (CCS) and then, based on these initial positive results, further application in patients with ACS has been attempted.
Although evidence supporting the use of coronary physiology guidance in ACS management is increasing, caution should be exercised in the interpretation of coronary physiology indices in this setting, given their potential pitfalls. This article summarises the evidence of the role of the main coronary physiology indices in ACS, focusing on five different clinical practice scenarios: STEMI with disease only in the infarct-related artery (IRA), STEMI with multivessel disease (MVD), non-ST-elevation ACS (NSTE-ACS) with unclear culprit lesion and MVD, NSTE-ACS with well-defined culprit lesion and MVD, and MI with non-obstructive coronary artery disease (MINOCA).
ST-elevation MI: The Infarct-related Artery
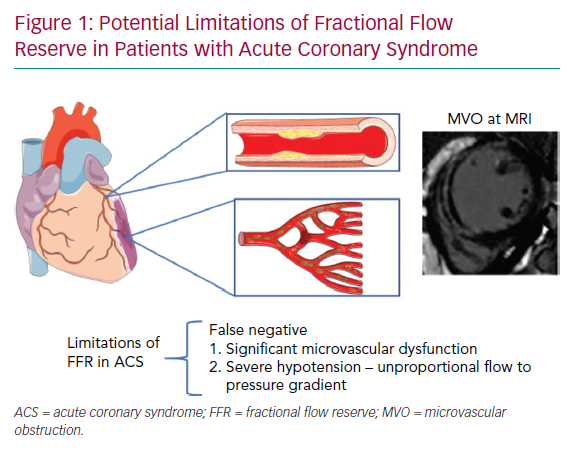
Usually, in STEMI undergoing primary PCI (PPCI), the IRA can be easily identified because of its acute angiographic characteristics, compatible with the clinical presentation. In this particular scenario, there is no need for physiological guidance to decide whether revascularisation is necessary. Moreover, the accuracy of epicardial functional indices such as FFR may be hampered by the significant degree of microvascular dysfunction observed in nearly 50% of cases, as discussed in detail below (Figure 1).
Nevertheless, coronary physiology can still play an important role in the assessment of the downstream microcirculatory function of the IRA, providing prognostically relevant information and identifying patients at high risk of suboptimal reperfusion who are eligible for additional novel therapies. The IMR, a pressure wire thermodilution-derived index supported by a large body of evidence, is the index of choice to assess the microvascular function in the IRA because of its ability to offer a reasonable compromise between accuracy and feasibility.2 In patients with STEMI, a cut-off value of IMR ≥40 has been associated with poor prognosis and more extensive myocardial injury.3
Microvascular Damage in the STEMI Infarct-related Artery
Within less than an hour of ischaemia in the territory of the IRA, oedema develops from structural alterations to cardiomyocytes, resulting in cardiomyocyte death after the first 3 hours. PCI is able to restore coronary blood flow in the IRA but may also have detrimental effects on the microcirculation, causing dislodgement of atherothrombotic debris and distal embolisation.3 Although endothelial cells are more resilient to ischaemia than cardiomyocytes, prolonged ischaemia eventually also results in endothelial dysfunction. As a consequence, capillary permeability is initially increased with oedema formation. Furthermore, endothelial dysfunction leads to impaired vasomotion, stasis and release of deleterious substances such as vasoconstrictors, inflammatory cytokines and reactive oxygen species. The final consequences of these processes are microvascular obstruction (MVO) and haemorrhage.4
It is well established that intramyocardial haemorrhage (IMH) and MVO are closely associated. However, IMH reflects a more irreversible degree of myocardial damage than MVO, which can instead shrink and eventually resolve at follow-up. MVO assessed by cardiovascular MRI (CMR) is an independent predictor of worse outcome regardless of infarct size, and patients with larger MVO are more likely to develop heart failure, leading to an increase in mortality. Thus, MVO represents a potential therapeutic target.
Invasive coronary physiology, and specifically IMR, predict the occurrence of MVO and provides important information regarding a patient’s prognosis and management, especially when CMR is unavailable or impractical.5
Temporal Changes in Coronary Physiology in the Infarct-related Artery
Cuculi et al. assessed the changes in coronary physiology over time after STEMI.6 In that study, 43 STEMI patients underwent physiological assessment of the IRA at the time of the PPCI, at day 1 and at the 6-month follow-up. Notably, the resting coronary flow, estimated via thermodilution, did not change over time after STEMI. Conversely, the hyperaemic coronary flow increased significantly at follow-up (coronary flow reserve [CFR] 1.8 ± 0.9 versus 3.1 ± 1.1; p<0.001). Consistently, IMR decreased progressively after STEMI, being 37.0 ± 22.3 after PPCI, 30.6 ± 21.4 at day 1 and 24.0 ± 22.0 at 6 months (p=0.002).
Interestingly, the epicardial coronary physiology in the IRA also showed significant variations over time. In particular, FFR decreased from 0.93 ± 0.06 after PPCI to 0.92 ± 0.06 at day 1 and 0.89 ± 0.06 at 6 months (p<0.001). In contrast, resting coronary physiology estimated by the baseline ratio of distal coronary pressure (Pd) to aortic pressure (Pa) did not change significantly over time (after PPCI: 0.96 ± 0.04; day 1: 0.95 ± 0.05; 6 months: 0.96 ± 0.04; p=0.22).6
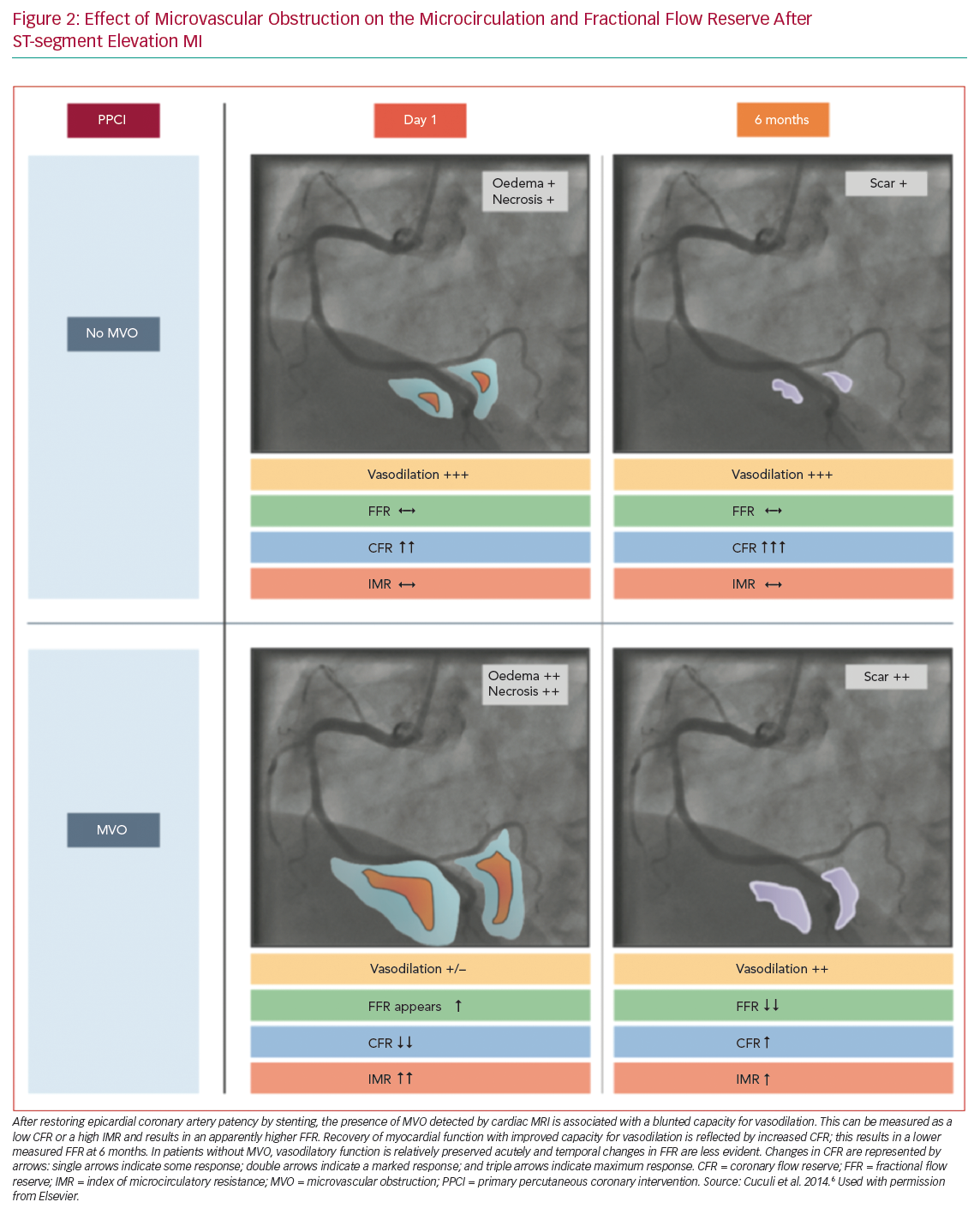
Notably, FFR variations over time were significant in patients with evidence of MVO at CMR (mean FFR 0.94 ± 0.04 versus 0.88 ± 0.06; p=0.006), but not in patients without MVO (0.94 ± 0.05 versus 0.93 ± 0.04; p=0.21; Figure 2).6
These interesting findings suggest that the coronary microcirculation generally recovers after STEMI in the IRA and tends to normalise 6 months after STEMI. The hyperaemic response to adenosine is blunted in the IRA, especially in patients with evidence of MVO. Therefore, the reliability of FFR in the acute phase of STEMI is questionable in the territory of the IRA. Whether the new adenosine-free indices can be used in the IRA in the setting of a recent STEMI is not clear, and further studies are needed.
Prognostic Value of Coronary Physiology After STEMI
An increasing body of evidence provides insights into the prognostic value of invasive physiology assessed at the time of PPCI with regard to acute and final infarct size, MVO, residual systolic function and clinical outcome after STEMI.
IMR at completion of PPCI has been associated with the extent of MVO (rho=0.29, p=0.002) and infarct size in the subacute phase after STEMI (rho=0.21, p=0.03) and at the 6-month follow-up (rho=0.43, p=0.001).5 In addition, post-PCI IMR ≥40 has been associated with higher risk of mortality and readmission for heart failure.7 Moreover, IMR ≥40 has shown excellent performance in predicting major in-hospital cardiac complications after PPCI (area under the curve [AUC] 0.90; 95% CI [0.85–0.93]).8 In addition, when measured before stenting, IMR can detect patients at high risk of suboptimal myocardial reperfusion who are candidates for additional therapies.9
A preserved vasodilatory capacity, reflecting an intact and functional coronary microvasculature, is an important predictor of myocardial functional recovery at 6 months after STEMI. The resistive reserve ratio (RRR) has been proposed to assess the vasodilatory capacity of the coronary circulation, and is calculated as the ratio between the baseline microcirculatory resistance (BMR) and the hyperaemic microcirculatory resistance expressed as IMR.10 Recently, it was demonstrated that RRR had incremental prognostic value in a small cohort of STEMI patients undergoing PPCI. In particular, patients with impaired RRR (<1.98) at completion of PPCI showed larger MVO (3.5 [0.0–5.9]; p=0.026), larger infarct size at 6 months (22.7 [10.2–35.0] versus 8.8 [6.9–12.3]; p=0.006) and a lower myocardial salvage index (34.0 [22.0–59.2] versus 53.2 [37.7–71.0]; p=0.032) than patients with preserved RRR.11
Numerous strategies have been developed to prevent or reduce the severity of microcirculatory dysfunction and MVO in patients with STEMI. In particular, the efficacy of intracoronary fibrinolytic therapy in STEMI patients after PPCI has been investigated, with controversial results.
Sezer et al. studied the effects of adjunctive low-dose intracoronary streptokinase given after PPCI in 41 STEMI patients.12 Of note, the treatment was effective in reducing IMR (16.29 ± 5.06 versus 32.49 ± 11.04; p<0.001) and increasing CFR (2.01 ± 0.57 versus 1.39 ± 0.31; p=0.002) compared with controls.12
In a larger study, patients who received adjunctive intracoronary streptokinase after PPCI demonstrated smaller infarct size (22.7% versus 32.9%; p=0.003) and better left ventricle ejection fraction (LVEF; 57.2% versus 51.8%, p=0.018) compared with controls.13
Conversely, McCartney et al. recently reported that patients who were randomised to receive low-dose intracoronary alteplase after PPCI did not differ from controls in terms of MVO at CMR (estimated difference 0.29%; 95% CI [−0.76–1.35%]; p=0.74) and presented similar clinical outcomes.14
Among the procedural techniques available to reduce microvascular and myocardial injury after STEMI, pressure-controlled intermittent coronary sinus occlusion (PiCSO; Miracor Medical) has been reported by us to reduce IMR (24.8 [18.5–35.9] versus 45.0 [32.0–51.3]; p<0.001) and infarct size at 6 months after STEMI (26% [20.2–30.0] versus 33.0% [28.0–37.0]; p=0.006) compared with controls.9 Intermittent occlusion of the coronary sinus allows redistribution of the coronary blood flow in under-perfused areas when the balloon is inflated and washing out of cellular debris and oedema fluid on balloon release, leading to relief of MVO.
Further details regarding available pharmacotherapy and procedural techniques to prevent and treat microcirculatory impairment in STEMI have been reported elsewhere.15
STEMI with Multivessel Disease: The Non-culprit Artery
More than 50% of patients presenting with STEMI have MVD.16 Recent evidence supports complete revascularisation compared with a culprit-only approach in patients with STEMI and MVD. The Complete vs Culprit-only Revascularization to Treat Multi-vessel Disease After Early PCI for STEMI (COMPLETE) trial demonstrated a significant benefit in terms of cardiovascular death and MI in a large population of STEMI patients who underwent complete revascularisation (HR 0.74; 95% CI [0.60–0.91]; p=0.004).17
The functional assessment of non-culprit lesions has been questioned because of concerns related to the status of the microvasculature in remote myocardial territories, with potential detrimental effects on the reliability of FFR or the instantaneous wave-free ratio (iFR). Numerous studies have addressed this question, and they generally favour the use of physiology to guide revascularisation of the non-culprit.
Fractional Flow Reserve and Instantaneous Wave-free Ratio Assessment of the Non-culprit Lesion
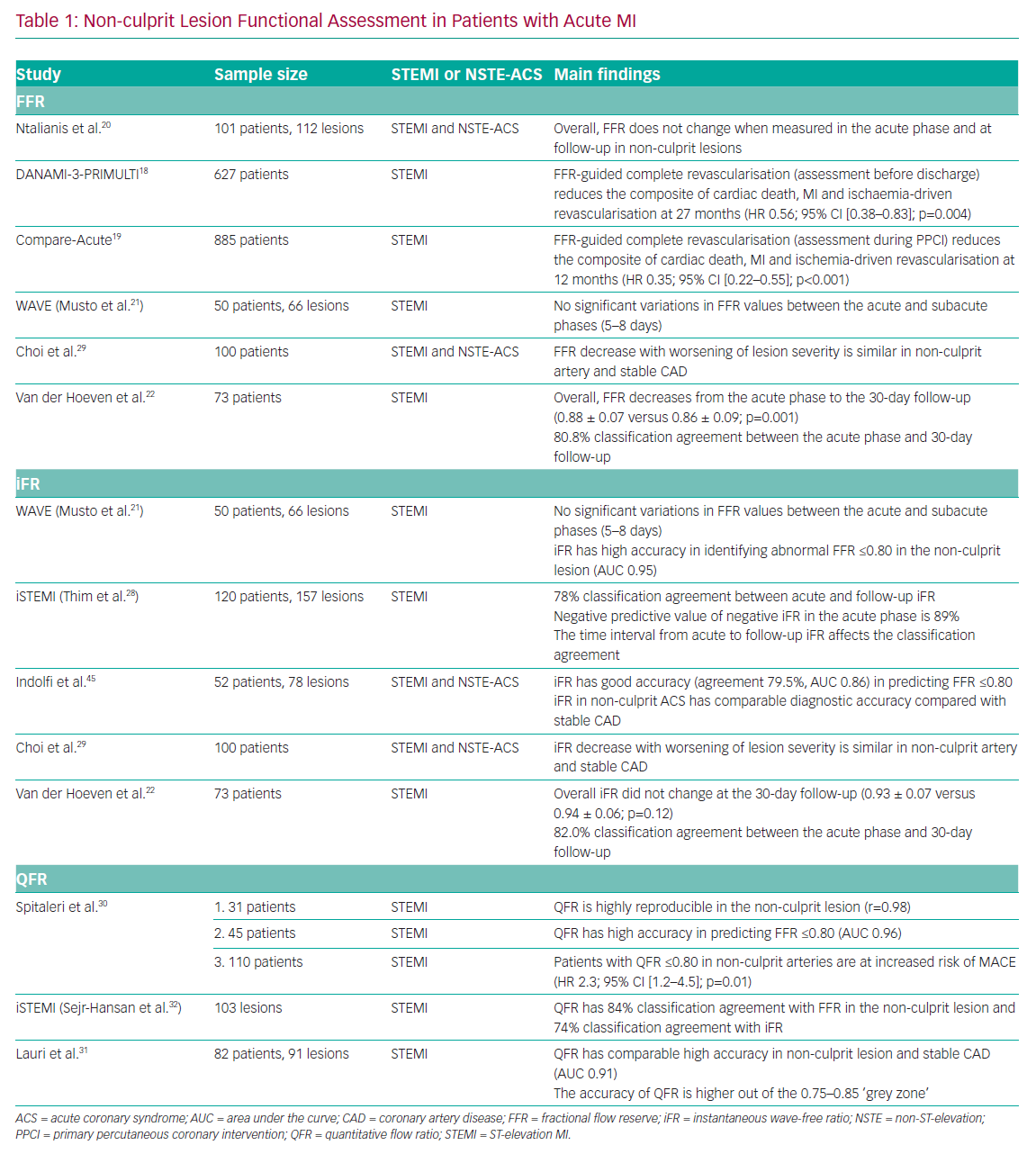
The Primary PCI in Patients With ST-elevation Myocardial Infarction and Multivessel Disease: Treatment of Culprit Lesion Only or Complete Revascularization (DANAMI-3-PRIMULTI) and Comparison Between FFR Guided Revascularization Versus Conventional Strategy in Acute STEMI Patients With MVD (CompareAcute) trials demonstrated the efficacy of FFR-guided complete revascularisation in patients presenting with STEMI.18,19 Interestingly, in the CompareAcute trial, functional assessment of the non-culprit lesions was performed during the PPCI procedure, whereas in the DANAMI-3-PRIMULTI trial the assessment was performed before discharge in a staged manner. Notably, both trials demonstrated the superiority of FFR-guided complete revascularisation compared with the culprit-only approach (Table 1).
The feasibility of FFR assessment of non-culprit lesions in patients with acute MI was assessed by Ntalianis et al.20 In that study, the authors found no overall significant difference in FFR values at follow-up compared with the acute phase. However, the heterogeneity of the study population has to be taken into consideration when interpreting the results, particularly with regard to the mixed clinical presentation (both STEMI and NSTE-ACS) and the time of follow-up (ranging from
4 to 128 days). However, the Instantaneous Wave-Free Ratio and Fractional Flow Reserve for the Assessment of Non Culprit Lesions in Patients With ST-segment Elevation Myocardial Infarction (WAVE) study also showed no significant variations in FFR in the non-culprit artery, even though the follow-up period was limited to 5–8 days after STEMI.21
Recently, a substudy of the Reducing MicroVascular Dysfunction in Revascularized STEMI Patients by Off-target Properties of Ticagrelor (REDUCE-MVI) trial demonstrated that CFR measured in the non-culprit vessel significantly increases (2.9 ± 1.4 versus 4.1 ± 2.2; p<0.001) and the IMR tends to decrease 1 month after the index procedure (18.0 [13.5–27.0] versus 14.5 [11.0–21.0]; p=0.6).22 Interestingly, the authors of that study observed a blunted hyperaemic response to adenosine in the acute phase of STEMI measuring Pd variations and RRR (3.4 ± 1.7 versus 5.0 ± 2.7; p<0.001). Consistent with these observations, FFR decreased significantly in the non-culprit vessel at the 1-month follow-up (0.88 ± 0.07 versus 0.86 ± 0.09; p=0.001), maintaining a classification agreement of 80.8% between the acute phase and follow-up assessment.
Notably, a blunted haemodynamic response detected in the non-culprit artery was associated with larger infarct size and worse LVEF after STEMI.22 If the reduced hyperaemic flow in the IRA can be explained primarily by the presence of infarct-related microvascular injury, this phenomenon is less well characterised in the non-culprit artery. It is known that the sensitivity of purinergic adenosine receptors is reduced in the remote myocardium in the acute phase of STEMI. Moreover, increased neurohumoral activation and extravascular compression secondary to myocardial oedema may play a role in the acute blunted hyperaemic response to adenosine.23,24
Nevertheless, Mejía-Rentería et al. recently observed that the hyperaemic flow was preserved in the subacute phase of MI, supporting the use of FFR in this setting.25 Notably, IMR (15.6 [10.4–21.8] versus 16.7 [11.6–23.6]; p=0.56) and RRR (3.1 ± 2.1 versus 3.7 ± 2.2; p=0.12) were similar in non-culprit lesions compared with a matched cohort of stable patients, whereas CFR was lower in the non-culprit lesions (1.77 [1.25–2.76] versus 2.44 [1.63–4.00]; p=0.018). Interestingly, the reduction in CFR was primarily driven by an increase in resting coronary flow (rest mean transit time 0.58 s [0.32–0.83] versus 0.65 s [0.39–1.20]; p=0.045).25 This observation may have implications for adenosine-free ischaemic indices in non-culprit vessels.
In particular, a tendency for overestimation of lesion severity was observed in the Nonculprit Stenosis Evaluation Using iFR in Patients with STEMI (iSTEMI) study using iFR. Notably, a similar trend was observed in different clinical settings where baseline coronary flow is markedly increased.26,27 In the iSTEMI study, the classification agreement between the acute phase and follow-up iFR values was modest (78%) and inferior compared with that for FFR.28 Conversely, in the study by van der Hoeven et al., iFR presented a similar classification agreement between acute and 30-day assessment to that obtained for FFR (82.2%).22
In conclusion, FFR-guided assessment and treatment of non-culprit lesions is supported by pathophysiological and randomised data. Less extensive experience supports the use of iFR in this clinical scenario, but iFR guidance of non-culprit lesions in a practice similar to the DANAMI-3-PRIMULTI and CompareAcute trials will require additional research and, ideally, a randomised control trial.21,22,28,29
Angiography-derived Functional Assessment of the Non-culprit Lesion
Recently, the quantitative flow ratio (QFR), a novel angiography-derived index, has been proposed to functionally assess non-culprit lesions in ACS patients. QFR in the non-culprit lesion has demonstrated high reproducibility between the acute and sub-acute phases of STEMI (r=0.98; 95% CI [0.96–0.99]; mean difference 0.004 [−0.027–0.34]) and high accuracy (AUC 0.96; 95% CI [0.89–0.99]) in predicting an abnormal FFR value (≤0.80).30
Similarly, Lauri et al. demonstrated the feasibility of performing QFR analysis retrospectively in patients with STEMI undergoing PPCI.31 In that study, the authors observed a high accuracy of QFR (AUC 0.91; 95% CI [0.85–0.97]) in predicting an abnormal FFR (≤0.80), especially when QFR is out of a receiver operating characteristic (ROC)-defined grey zone (0.75–0.85). When a hybrid QFR–FFR approach was used, measuring FFR only when QFR is in the grey zone, an overall 96.7% classification agreement was obtained, avoiding further invasive diagnostic procedures in the non-culprit vessels in 58.5% of patients.32 A post hoc analysis of the iSTEMI study revealed good accuracy of QFR in predicting an abnormal FFR (84%; 95% CI [76–90]) in the non-culprit artery and moderate accuracy compared with an abnormal iFR (74%; 95% CI [65–83]).32 Notably, Spitaleri et al. showed that patients with untreated non-culprit lesions with QFR ≤0.80 were at higher risk of adverse clinical events (HR 2.3; 95% CI [1.2–4.5]; p=0.01).30 Angiography-derived indices, and in particular QFR, may find a role in the simplification of ACS management, and further randomised data are warranted to confirm these preliminary findings.
NSTE-ACS with Clear Culprit Lesion and Multivessel Disease
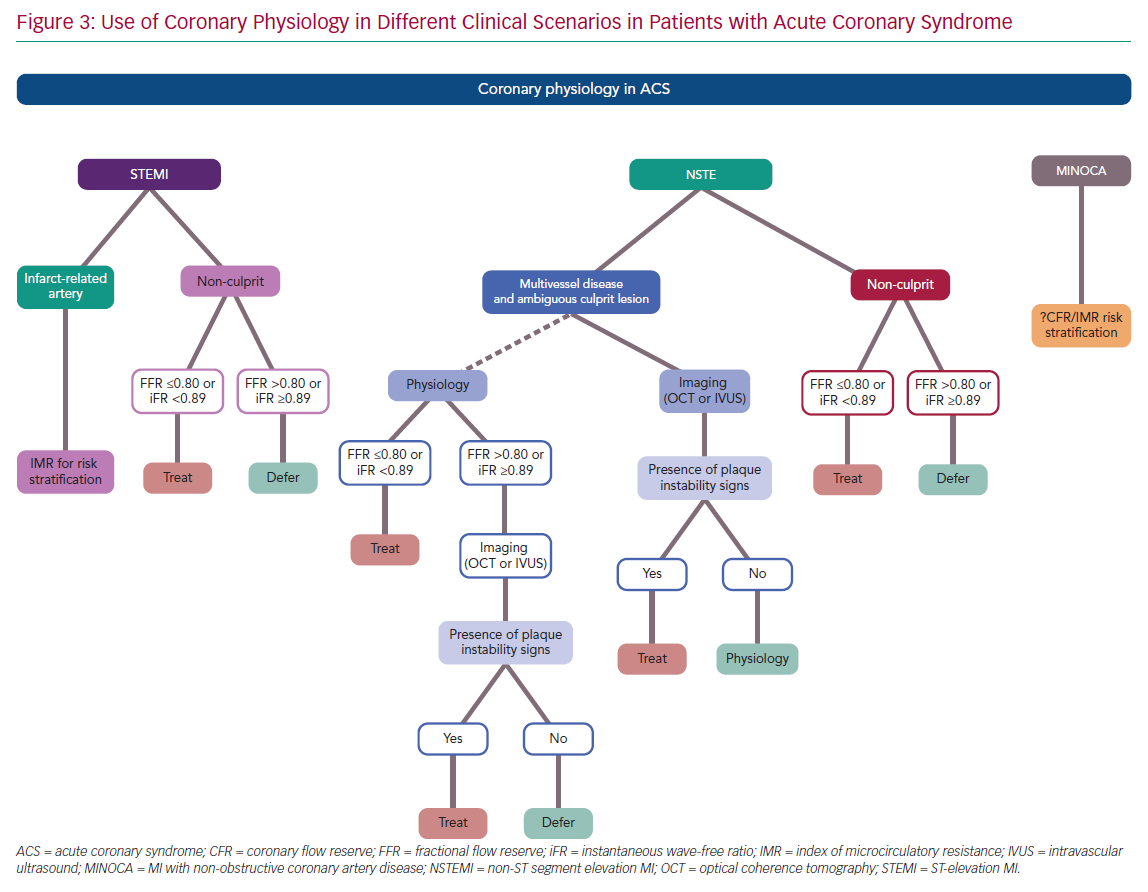
In presence of a clear infarct-related lesion and bystander MVD, the same information reported for the STEMI non-culprit lesions can be applied to NSTE-ACS patients. In particular, FFR and iFR have been used in this setting with favourable outcomes and should be considered in the presence of angiographic intermediate lesions (Figure 3).
Microvascular Vasodilatory Capacity and Hyperaemic
Physiology in NSTE-ACS
The question of a reliable achievable maximal hyperaemia in patients with NSTE-ACS has been explored by Layland et al. using thermodilution-derived RRR.10 Notably, the vasodilatory response of the coronary microcirculation was comparable between patients with NSTE-ACS and stable coronary artery disease (RRR 2.5 [1.6–3.9] versus 2.8 [1.7–4.8]; p=0.61). These findings confirm the preserved capacity of the coronary microcirculation to achieve maximal hyperaemia in NSTE-ACS and are reassuring about the reliability of hyperaemic physiology in NSTE-ACS.10
The Fractional Flow Reserve Versus Angiography in Guiding Management of Optimize Outcomes in Non-ST-Elevation Myocardial Infarction (FAMOUS-NSTEMI) trial demonstrated the safety and feasibility of FFR measurements in NSTE-ACS.33 An interesting FAMOUS-NSTEMI substudy demonstrated that FFR has a 92% diagnostic accuracy (positive predictive value 76%, negative predictive value 97%) in detecting significant perfusion abnormalities in matched territories at stress CMR (AUC 0.93; 95% CI [0.90–0.99]).34
IMR has been less extensively studied in the NSTE-ACS setting compared with STEMI. In the study of Layland et al., pre-PCI IMR values in NSTE-ACS patients did not differ significantly from those in stable angina patients (22.73 ± 11.36 versus 18.26 ± 9.15; p=0.1), but were significantly lower than in STEMI IRA (22.73 ± 11.36 versus 36.51 ± 35.7; p=0.01).10 Murai et al. investigated the prognostic value of post-PCI coronary physiology in 83 patients with NSTE-ACS. Notably, IMR and CFR <2, but not FFR, were significantly associated with major adverse cardiac events (MACE) at 20.7 months of follow-up.35 Multivariate analysis revealed that high IMR was an independent predictor of MACE in this NSTE-ACS cohort (HR 1.03; 95% CI [1.01–1.05]; p=0.001).35
NSTE-ACS with Ambiguous Culprit Lesion and Multivessel Disease
When coronary intervention is deferred based on coronary physiology, patients presenting with ACS have a higher risk of MACE at follow-up than stable coronary artery disease patients.36,37 The Fractional flow reserve versus Angiography for Multivessel Evaluation (FAME) trial enrolled 328 patients with NSTE-ACS, of whom 150 were randomised to FFR-guided PCI. Notably, the risk of MACE at 2 years was higher in the ACS group than stable patients (21.3% versus 16.4).36
Recently, a combined analysis of the Functional Lesion Assessment of Intermediate Stenosis to Guide Revascularisation (DEFINE-FLAIR) and Instantaneous Wave-Free Ratio Versus Fractional Flow Reserve in Patients With Stable Angina Pectoris or Acute Coronary Syndrome (iFR SWEDEHEART) trials confirmed that the ACS presentation was associated with a higher incidence of MACE at 1 year in 2,130 patients with coronary lesions deferred based on ‘negative’ values of iFR or FFR (for stable coronary artery disease presentation, HR 0.61; 95% CI [0.38–0.99]; p=0.04).37 It remains unclear whether this higher rate of events registered in patients with ACS is related to an intrinsic higher risk in ACS patients or whether it reflects a ‘false negative’ physiological assessment.
In the setting of NSTE-ACS, the culprit lesion is often less obvious than in STEMI, especially when there are no specific angiographic features (e.g. intracoronary thrombus, ulceration, dissection), ECG changes or regional wall motion abnormalities. In this clinical scenario, a few aspects can be considered in the assessment of intermediate coronary lesions in patients with NSTE-ACS and ambiguous culprit plaque or artery.
In the IRA, postulating a plaque rupture and a preserved conduit vessel luminal area, FFR and iFR results may be above the ischaemic thresholds, even in case of an intact downstream microvasculature. Conversely, in case of extreme ACS-related microvascular dysfunction, physiological indices may be falsely elevated even in case of flow-limiting intraluminal disease.38
In patients with ACS, the optimal FFR cut-off for treatment deferral has been questioned, observing that the rate of MI or target vessel failure was 12.8% per year when FFR was 0.75–0.80, 10.0% per year when FFR was 0.80–0.85 and 6.2% per year for FFR values >0.90. Notably, such a trend was not observed in patients with stable angina.39
Given the theoretical limitations of FFR and iFR in case of an acute plaque event, physiology should be integrated with intracoronary imaging to detect the presence of plaque rupture, erosion or intracoronary thrombus. In particular, optical coherence tomography (OCT) or intravascular ultrasound (IVUS) should be considered as the first choice to guide revascularisation in case of uncertainty regarding the IRA in the setting of NSTE-ACS. If functional assessment by means of FFR or iFR is preferred by the operators, imaging should still be considered in the case of a borderline or negative result (FFR >0.80 or iFR ≥0.89; Figure 3).
MI with Non-obstructive Coronary Artery Disease
MINOCA is the term currently used to describe patients presenting with clinical features of an acute myocardial injury but with no evidence of obstructive coronary artery disease on coronary angiography, so that the direct cause for the clinical syndrome is not evident.40 MINOCA is not an uncommon condition and has been reported in 5–15% of patients with suspected MI admission. MINOCA encompasses a wide variety of aetiological mechanisms that could be differentiated into epicardial and microvascular. Regional wall motion abnormalities limited to a single coronary artery territory suggest an epicardial mechanism that is caused primarily by coronary plaque disease, spasm or dissection. Conversely, regional wall motion abnormalities extending to more than one epicardial coronary artery territory suggest a microvascular mechanism that is primarily caused by takotsubo syndrome, myocarditis, coronary microvascular spasm and coronary embolism.
Data on the role of coronary physiology in MINOCA are scarce and stem from small pilot studies and case reports.
During the acute phase of takotsubo syndrome, significant microcirculatory dysfunction with a global distribution pattern has been described, with a tendency towards normalisation during the recovery phase. Possible underlying mechanisms of the temporary disrupted perfusion and myocardial stunning include diffuse vasoconstriction due to catecholamine-induced alpha-adrenoceptor stimulation in resistance arteries, as well as endothelial dysfunction and inflammation.41–43
Although there is currently no clinical indication for coronary physiology assessment in MINOCA, it is possible that the assessment of microvascular dysfunction may lead to a better risk stratification and personalised treatment.44 Understanding coronary physiology in MINOCA and implementation of targeted therapies to improve prognosis represent important challenges for future dedicated research.
Conclusion
Coronary physiology provides useful information in guiding the management of patients with ACS, as summarised in Figure 3. There is usually no need for epicardial functional assessment of the IRA in STEMI. Moreover, a significantly impaired microcirculatory function is detected in more than 50% of STEMI patients, limiting the value of FFR or iFR in the IRA. Conversely, IMR has emerged as an important tool to stratify the clinical risk of adverse events or adverse left ventricle remodelling in STEMI. In addition, increasing data suggest a potential role of IMR in identifying patients who may benefit from additional therapies on top of standard approaches with stenting to prevent suboptimal myocardial reperfusion.
In the setting of NSTE-ACS, physiology should be integrated with intracoronary imaging in the case of an ambiguous IRA (Figure 3), and OCT or IVUS should be used to detect signs of plaque instability in case of ‘negative’ functional assessment. Conversely, an increasing body of evidence supports the use of coronary physiology in the non-culprit lesion of both STEMI and NSTE-ACS, and complete revascularisation is recommended by the latest European Society of Cardiology guidelines.1
Finally, coronary physiology assessment in patients with MINOCA represents an interesting field for future dedicated research.