Dr Eugene Braunwald is one of the most eminent figures in modern cardiology and has authored more than 1,000 peer-reviewed publications. His work has increased current understanding of congestive heart failure, coronary artery disease and valvular heart disease. Among his notable works are the Thrombolysis in Myocardial Infarction studies, which elucidated the pathophysiology of acute myocardial infarction. He is also responsible for Braunwald’s Heart Disease, the most widely read textbook of cardiology in the world.
Dr Braunwald began by discussing his early work on the determinants of myocardial oxygen consumption.1,2 The idea that early reduction of myocardial oxygen demands and improvement of coronary perfusion might reduce infarct size dates back to the early 1970s.2 This concept was advanced further in 1976 with the first publication of coronary reperfusion after coronary thrombolysis,3 and in 1981 when it was proven that thrombolytic reperfusion salvaged myocardial tissue.4 These findings led to the establishment of the Thrombolysis in Myocardial Infarction (TIMI) study group by the National Institutes of Health in 1985. Among this group’s most important developments was the TIMI Risk Score, which assesses the risk of death and ischaemic events in patients experiencing unstable angina. The next major advance in the relationship between acute myocardial infarction (AMI) and heart failure (HF) was in 1987, when Pfeffer et al. found that the haemodynamic profile of chronic HF secondary to myocardial infarction (MI) could be pharmacologically altered in rats, but the improvements were significantly diminished in hearts with large infarcts.5 This finding led to the first report of post-AMI cardiac remodelling in 1990.6 These findings established the pathophysiological basis for the progression to HF in patients with AMI and were a key milestone toward the development of reperfusion strategies, including primary percutaneous coronary intervention (PCI). A 2013 study found that in Medicare beneficiaries, hospitalisation for HF following AMI decreased only slightly from 1998 to 2010 and that 1-year mortality remained essentially unchanged.7 Until recently, it was not known whether the extent of coronary artery disease (CAD) was associated with the occurrence of HF after AMI. In a recent paper, however, atherosclerotic burden was found to be an indicator of post-MI HF regardless of HF type and independent of recurrent MI.8 While it has been long established that the use of PCI to treat infarct arteries improves prognosis, in 2013 Wald et al. demonstrated the value of preventive PCI of non-infarct arteries with major stenosis in patients with ST-elevation myocardial infarction and multivessel CAD undergoing infarct artery PCI. Preventive PCI significantly reduced the risk of major adverse cardiac events in these patients.11
Dr Braunwald then turned his focus to the challenges faced in the management of AMI complicated by cardiogenic shock (CS). An acute ST-elevation myocardial infarction complicated by CS is associated with high mortality and CS is the leading cause of death in patients with AMI. A recent study used the Cath-PCI Registry® to evaluate trends in demographics, clinical characteristics, management strategies and in-hospital outcomes in patients with CS-AMI who underwent PCI from 2005 to 2013. The study found that, despite the evolution of medical technology and contemporary therapeutic measures, in-hospital mortality of AMI-CS patients continues to rise.9 There is a need to improve outcomes in these patients. Another recent study found that survivors of AMI-CS had a higher risk of death and/or hospitalisation during the first year after discharge compared to those without CS, and that the risk was highest in the early post-discharge period (first 60 days). After this time, the prognosis was similar in patients with or without CS.10
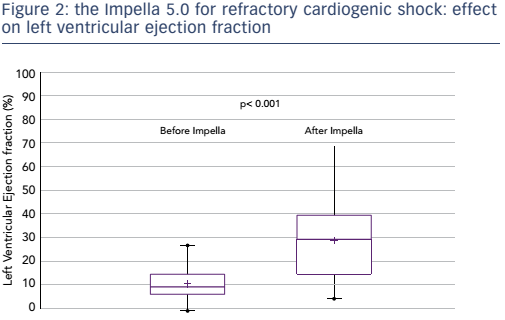
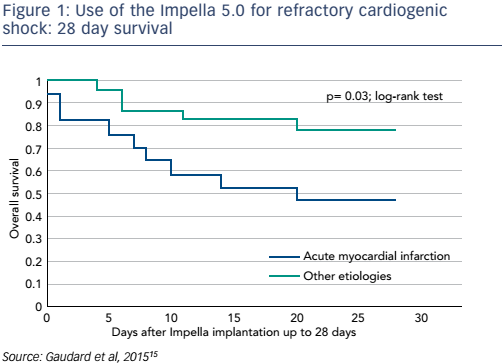
The failure of pharmacological treatments to maintain adequate perfusion and to prevent irreversible end-organ failure in many patients with CS has led to attempts to improve outcomes by mechanical circulatory support (MCS) devices. Until recently, initiation of MCS with an intra-aortic balloon had a class I recommendation for the treatment for CS-AMI and had become widely used. However, a 2012 study found that use of the intra-aortic balloon did not reduce 30-day mortality in patients with MI and CS.12 In recent years, the use of percutaneously-inserted left ventricular assist devices prior to PCI has become increasingly important. The TandemHeart™ (CardiacAssist Inc) has proven beneficial in patients in severe CS refractory to intra-aortic balloon pump and vasopressor therapy, but CS patients still had worse outcomes in terms of mortality than those without CS.13 A 2014 study showed that early use of the Impella® 2.5 prior to PCI was associated with more complete revascularisation and improved survival in the setting of refractory CS-AMI.14 The Impella 5.0 has also been studied for CS resulting from AMI, dilated cardiomyopathy and postcardiotomy cardiac failure: this has demonstrated impressive outcomes in terms of mortality (see Figure 1). Furthermore, following removal of the Impella, patients’ left ventricular ejection fraction improved significantly (p<0.001) when compared to baseline (see Figure 2).15
As a result of these findings, the 2015 clinical expert consensus statement on the use of percutaneous MCS devices in cardiovascular care stated that percutaneous MCS provides superior haemodynamic support compared to pharmacological therapy. The guidelines also stated that in profound CS, MCS using intra-aortic balloon is less likely to provide benefit than continuous flow pumps. Dr Braunwald discussed the importance of early placement of an appropriate MCS as being key in patients in CS who fail to stabilise or quickly show improvement after initial intervention. Furthermore, MCS may be considered for patients undergoing high-risk PCI.16
Dr Braunwald emphasised the need to develop strategies to reduce reperfusion injury, which is a major contributor to the final myocardial infarct size. There is also a need to reduce myocardial oxygen demands and to initiate early pharmacological treatment to reduce ventricular size and diminish wall stress. Secondary prevention of recurrent AMI is also important. This should involve intensive reduction of low-density lipoprotein through the use of proprotein convertase subtilisin/kexin type 9 inhibitors to reduce recurrent AMI.
Dr Braunwald concluded by emphasising that early application of these new MCS devices is needed in AMI-CS and acute, decompensated HF. Brief, temporary MCS should be applied for a longer period and may become a bridge to surgically-implanted durable left ventricular assist devices, biventricular assist devices, cardiac transplantation and recovery.