Over the past 30 years, several percutaneous transcatheter technologies and devices for interventions in structural heart diseases (SHDs) have been introduced (see Table 1). There are numerous technologies that are in development or are currently being used to treat patients with SHD that use transcatheter techniques. The variety of percutaneous treatment approaches has led to a revolution and evolution in clinical care. The past few years have seen a greater application of novel, catheter-based treatments for SHDs. Many of these non-surgical catheter-based interventional procedures have proven effective, which has consequently resulted in a greater acceptance among physicians and patients alike.
The rapid growth of minimally invasive interventional procedures for treating SHD has been accompanied by new challenges and demands on the imaging specialist. Fluoroscopy is inadequate for visualising cardiac valves, congenital and acquired defects. Soft tissue imaging and three-dimensional (3D) delineation of the heart structures is not feasible with fluoroscopy or standard contrast ventriculography. Consequently, additional imaging technology is needed to diagnose and precisely identify SHD in order to appropriately select patients for specific interventions and to support fluoroscopy in guiding procedures.
Three-dimensional transoesophageal echocardiography (TOE) and multi-slice computed tomography (MSCT) provide precise pre-treatment information on anatomic abnormalities, the exact localisation of the cardiac pathology, its severity and precise anatomic dimensions of the lesion.1,2 Such information facilitates procedural planning on the best ways to access and treat the SHD lesion. Moreover, detailed information is provided regarding the anatomy in relation to neighbouring structures.
New technologies for the treatment of SHD are proliferating; consequently, the number of interventions for SHD is increasing3–5 and the procedures are becoming more complex. This has produced a need for greater expertise in the evaluation and imaging of SHD. Due to these changes in the therapeutic armamentarium, interventional imaging appears to be becoming a requisite sub-speciality.
Imaging Modalities for Structural Heart Disease Procedures
Two-dimensional (2D) TOE (or alternatively 2D intracardiac echocardiography) in conjunction with fluoroscopy and cineangiography has been the standard imaging practice in most catheterisation laboratories; however, these imaging modalities are limited in the visualisation of soft tissue, complex 3D structures and their relationships. The introduction of 3D TOE, cardiovascular magnetic resonance (CMR) imaging and MSCT has overcome some of these limitations and, as a result, these additional modalities are being widely adopted in the process of selecting SHD patients, monitoring and guiding procedures, and assessing procedural results. The development and implementation of advanced cardiac imaging constitutes one of the key factors in the success of SHD interventions.6
Three-dimensional Transoesophageal Echocardiography
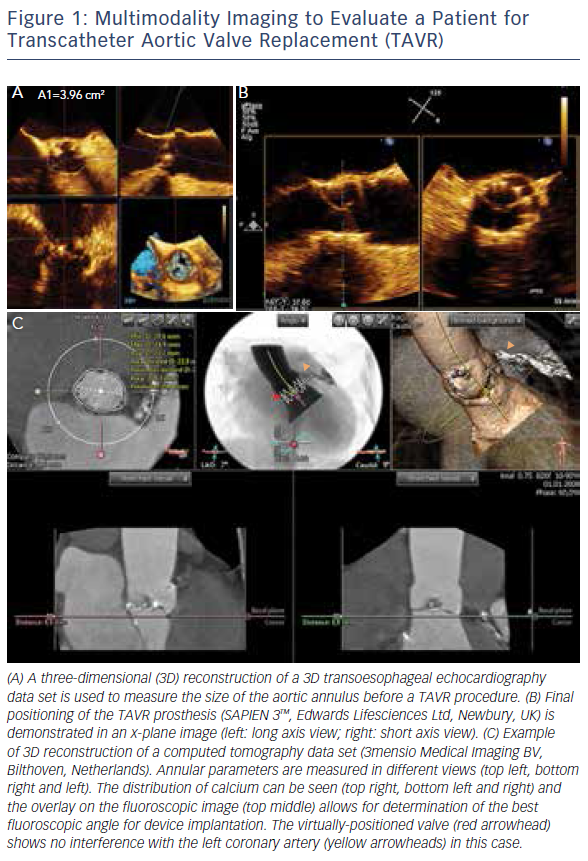
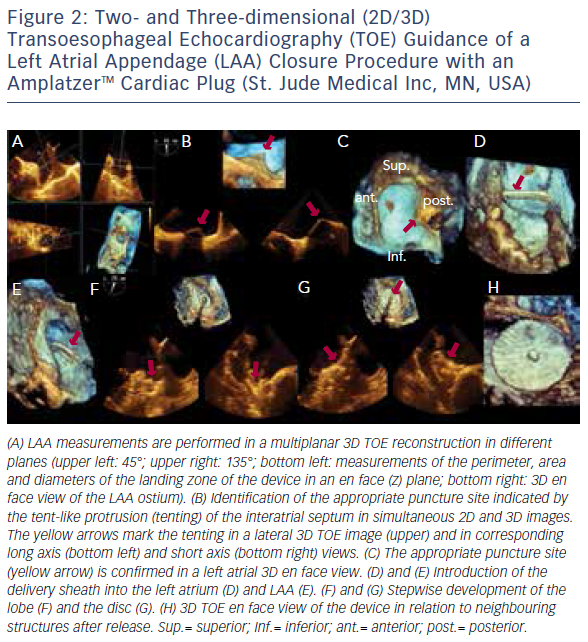
The introduction of 3D TOE has led to a major technological advance in echocardiographic imaging. Realtime 3D TOE imaging provides unique en face views and excellent detail of patients’ 3D anatomy and soft tissue structures. It also allows for the ‘live’ guidance of interventional procedures. Wires, catheters, sheaths, devices and target structures can be seen in one single view and in relation to each other, thus facilitating the guidance of standard and complex SHD interventions. While 3D TOE is gaining acceptance, it is still not available in all centres. Two-dimensional TOE has the advantage of providing higher resolution images, which can be helpful in specific situations where precise measurements are needed. Echo guidance with 2D in conjunction with 3D TOE is crucial and is recommended for many different kinds of mitral intervention,7–10 aortic and mitral paravalvular leak closure,10–13 transcatheter aortic valve replacement (TAVR) (Figure 1A and B),10,14–16 left atrial appendage (LAA) occlusion (Figure 2),10,17 atrial septal defect10,18–20 and patent foramen ovale closure10,21 as well as for percutaneous closure of ventricular septal defects.22
Multi-slice Computed Tomography and Cardiovascular Magnetic Resonance Imaging
Although TOE is the most widely used imaging modality, MSCT and CMR are helpful imaging techniques for the pre- and postprocedural assessment of anatomy, pathology and function of cardiac structures, device assessment, and for the detection of complications post-procedure.
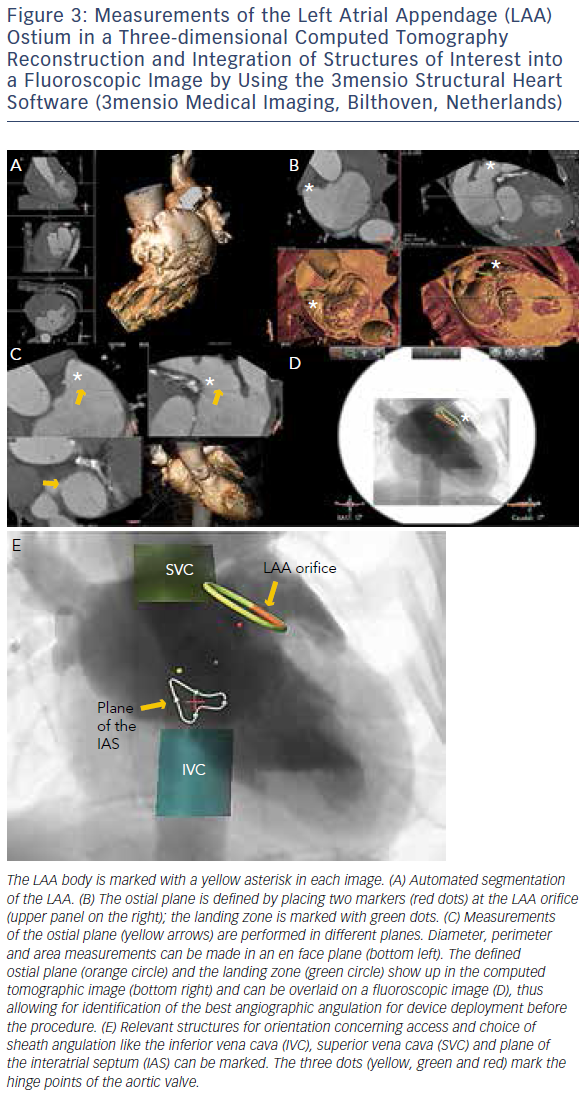
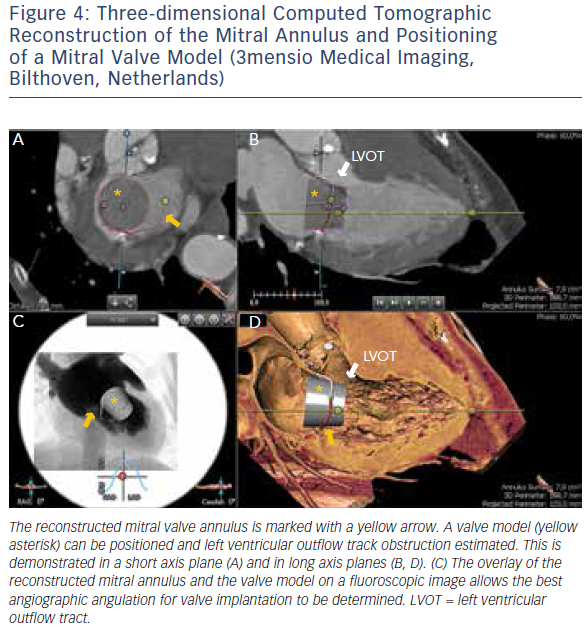
MSCT provides accurate data for sizing devices for TAVR (Figure 1C) and LAA occlusion (Figure 3A–D).2 Before a TAVR procedure, the diameters, areas and perimeters of the aortic annulus, sinuses of Valsalva and ascending aorta can be accurately determined. In addition, the location and distance of the coronary artery ostia from the aortic valve and bypass grafts can reliably be visualised. The amount and distribution of calcification can be identified. MSCT-based analysis of the aortic root and vascular access sites has become routine in the pre-procedural evaluation of patients for TAVR.23–28 In post-procedural assessment, MSCT has been beneficial in detecting complications like reduced aortic-valve leaflet motion in patients with bioprosthetic aortic valves.29 In addition, MSCT is useful for the evaluation of LAA anatomy and measurements regarding device selection, assessment of procedural success and longer-term outcomes.30,31 MSCT is also beneficial in other SHD procedures. It has been used to assess the function and anatomy of the mitral valve complex.32–34 As new mitral valve devices undergo clinical trials, MSCT will likely play a critical role in determining patient eligibility (see Figure 4 for an example of this), especially in the assessment of patients with mitral annular calcification.33,35 Investigators have shown that MSCT can be used to diagnose and differentiate interatrial shunts.36,37 In patients with paravalvular leaks, ECG-gated computed tomography angiography has proven useful in characterising the precise anatomy of the paravalvular leaks, thus facilitating appropriate occluder device selection.38,39
Limitations of MSCT include ionising radiation, a lower temporal resolution than TOE, and the inability to use it during interventional procedures. Nonetheless, due to the proven utility of MSCT in the context of SHD interventions, this imaging modality will likely play a role in the development of future sizing algorithms.
There is limited experience with CMR; however, it is an attractive alternative imaging modality in pre- and post-procedural evaluation of SHD, particularly in patients with renal failure40 and in patients with difficult echocardiographic windows.41 This non-invasive imaging modality allows for detailed visualisation of cardiac anatomy and functional assessment, including quantification of chamber size and volume, left ventricular function (systolic and diastolic), myocardial tissue characterisation and precise wall motion analysis without exposing the patient to ionising radiation.
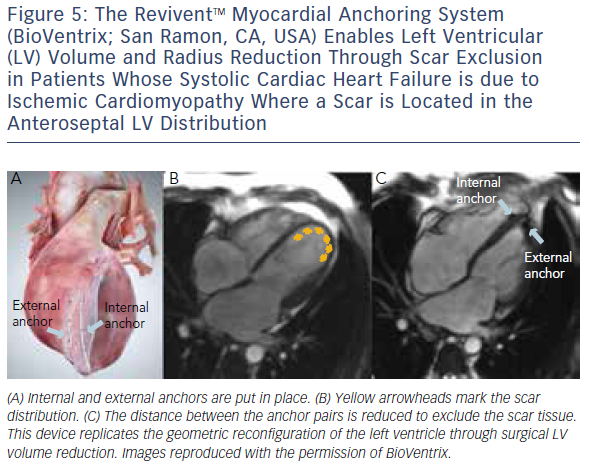
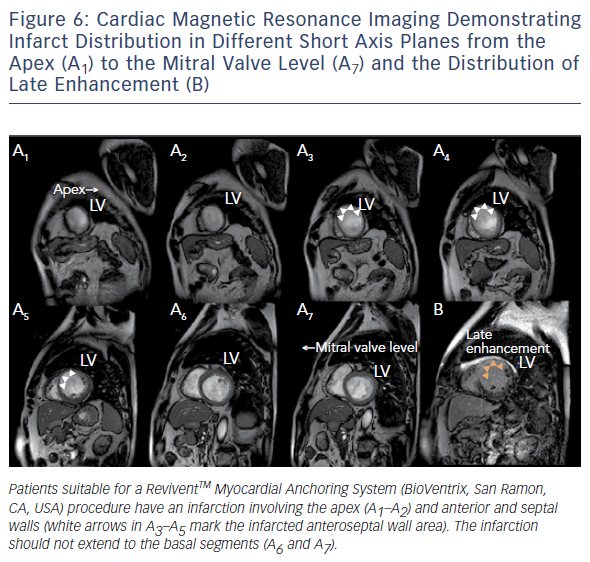
Similar to cardiovascular MSCT, CMR also provides imaging with excellent spatial resolution and can perform 3D multiplanar reconstruction. Figures 5 and 6 demonstrate the usefulness of CMR imaging in patient selection for the ReviventTM Myocardial Anchoring System (BioVentrix, San Ramon, CA, USA). There are, however, limitations to MSCT. These include prolonged examination times, dependence on the ability to perform adequate breath holds, and its limited use in patients with implanted pacemakers or defibrillators and during the device implantation procedure.
Integrated Multimodality Imaging
More recently, advances in software and hardware development have enabled the integration of various imaging modalities into a single data set, thus resulting in realtime fusion imaging after the separate acquisition of two image data sets. The side-by-side registration of data rendered by different non-invasive imaging modalities such as echocardiography, advanced computed tomography and magnetic resonance imaging technologies and fluoroscopy may overcome some of the limitations of each of the modalities when used as a sole imaging method. Such a multimodal imaging approach may thereby provide increased diagnostic and procedural accuracy by combining anatomical and functional information (see Figure 1C, Figure 3D and E and Figure 4C).42–48
Challenges during Structural Heart Defect Interventions
Currently, interventional transcatheter techniques are being used to treat patients with SHD, including those at high surgical risk. SHD interventions require specifically-designed diagnostic catheters, guiding catheters, guide wires, sheaths and dedicated implantation tools and devices. The visualisation of moving wires, catheters, sheaths and devices within the 3D space of the moving (beating) heart in relation to the target regions constitutes one of the major procedural challenges. In this context, 3D TOE imaging facilitates the manipulation and alignment of devices to the target lesion, thereby increasing the odds of achieving procedural success.
The dynamic and interactive nature of SHD interventions requires a multidisciplinary team with expertise in imaging and intervention. It is crucial that the imaging specialist and the interventionalist communicate with each other at all times during an interventional procedure to ensure optimal and safe deployment of the device. The orientation of echocardiography and fluoroscopy images differs significantly. TOE images are typically obtained through a relatively narrow imaging window through the oesophagus,49 whereas the rotation of the C-arm allows for the acquisition of multiple views of the same structure.35 Thus, the same structure is seen from different perspectives by the echocardiographer and the interventionalist. Identifying the same structure simultaneously on echocardiographic and fluoroscopic images is complicated, therefore the echocardiographer and the interventionalist must make sure that they are communicating effectively to ensure effective manoeuvring of devices to obtain the best possible results.50 As each imaging technique provides unique and supplemental information, the combination of multiple imaging techniques is helpful in precisely defining the anatomy and facilitating device deployment. Thus, the accurate description of anatomy, pathology and function and their effective communication between team members are key factors in a successful procedure. The use of realtime multimodality fusion imaging facilitates this process.42,43
The Evolution of Interventional Imaging as a New Sub-specialty
Performing SHD interventions is challenging; therefore accurate information on the exact location and anatomy of the target lesions and structures is required. Furthermore, imaging is important for determining the most suitable access for transcatheter procedures. This enables interventionalists to safely and accurately insert and position guide wires, catheters and dedicated devices during structural heart interventions.
Due to the complexity of the visual–spatial relationships, imaging specialists are key members of the interventional heart team. As with all cardiovascular procedures, experience is requisite to develop the skills necessary in the clinical setting. Imagers as well as interventionalists performing SHD interventions therefore require specific training on the use of each unique interventional device, as well as knowledge on the specific implantation requirements of these devices.3 Specific guidelines and recommendations, particularly for TOE monitoring of interventional procedures, have been produced by various cardiac societies.3,10,49,51,52 These documents emphasise the value of interventional imaging.
As there are numerous devices available for the treatment of SHD and more in development, substantial growth in the field is expected. Consequently, interventional imaging as a sub-specialty is likely to become even more important than it currently is and is likely to develop into a well defined and recognised sub-specialty.
Conclusion
SHD intervention is a burgeoning field. The sub-specialty of interventional imaging will likely develop out of an increased need for high-quality imaging. Imaging expertise constitutes a key factor in the decisionmaking process and in the management of patients with SHD in order to offer patients optimal outcomes from transcatheter interventions.