Out-of-hospital cardiac arrest (OHCA) is defined as the sudden absence of cardiac mechanical contractility with loss of signs of circulation that occurs within a community setting.1 OHCA affects more than half a million patients globally per year and is one the leading causes of death in developing countries.2 In the US, OHCA affects 350,000 patients per year and is the third leading cause of death.3–6 In the UK, the Ambulance Association reported that there were nearly 60,000 cases of OHCA in 2006, with cardiopulmonary resuscitation (CPR) attempted in less than half these patients.7
When the presenting rhythm is pulseless electrical activity or asystole, the underlying causes are often trauma, metabolic and electrolyte disturbance, drug overdose, subarachnoid haemorrhage, sepsis or pulmonary embolism.8,9 Patients with cardiac arrest and a rhythm that is suitable for defibrillation (i.e. VF or ventricular tachycardia), and where the arrest is witnessed, are more likely to have a cardiac aetiology and are known as the ‘Utstein comparator cohort’.10
Although improvements in prehospital care, exemplified in the ‘chain of survival’, remain central to improving outcomes after OHCA, there is now an increasing appreciation of the role of specialist interventional cardiological services and cardiac arrest centres.11 In this article, we review the contemporary management of OHCA with particular focus on interventional considerations in the cardiac catheterisation laboratory.
General Considerations
Conveyance to Centres: Should the Patient be Taken to a Specialist Centre with Cardiovascular Facilities?
There remains significant regional and temporal variation in outcomes after OHCA, and a combination of resources, centre experience and personnel could account for these disparities.12–19 This indicates that, as with other acute conditions, regionalisation of specialist services has the potential to improve short- and long-term clinical outcomes after OHCA.20,21 The International Liaison Committee on Resuscitation (ILCOR), American Heart Association and NHS England now recommend that all patients with OHCA should be transferred directly to specialist centres, known as cardiac arrest centres, for provision of emergency specialist cardiac services (including interventional cardiology) and experienced critical care services with access to targeted temperature management (TTM).22–24
It is important to note that there is significant variation in the expertise of emergency medical services globally and within health services, which will affect the development of pathways of care. Some emergency medical services are staffed only by paramedics, whereas others are staffed by emergency medicine physicians; in addition, the range of services provided before conveyance varies significantly. Furthermore, population density, prevalence of disease and transfer times for conveyance to a centre differ considerably, and these factors will have an effect on the delivery of protocols of care.
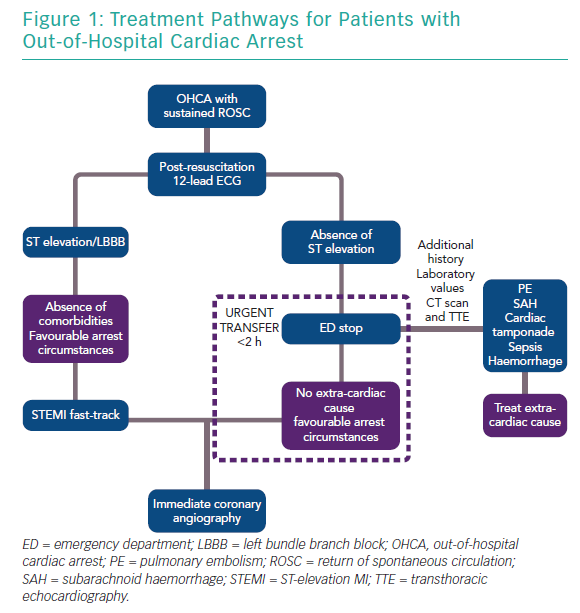
As patients with retained consciousness after return of spontaneous circulation (ROSC) have excellent survival with good neurological recovery (~98%), it is currently recommended that they should be treated as acute coronary syndrome patients without OHCA.25–27 The European Association of Percutaneous Cardiovascular Interventions (EAPCI) currently recommends that all patients with ST elevation (and favourable arrest circumstances, namely witnessed arrest, zero flow time <10 minutes and initial shockable rhythm) should be taken directly to a cardiac arrest centre.28
The current consensus for patients without ST elevation is that they are taken to any emergency department for evaluation of non-cardiac causes and, in the absence of such causes, are urgently transferred to a cardiac catheterisation laboratory, ideally within 2 hours (Figure 1). For patients without ST elevation, this process may lead to delays that could be detrimental, particularly in the presence of haemodynamic instability. It is also well known that patients with non-cardiac causes of OHCA, such as neurological or renal aetiologies, may exhibit ST changes on the 12-lead ECG. Therefore, it is important that cardiac arrest centres should have 24-hour access to CT scanning, expert neurosurgical care and be able to provide renal replacement therapy, which may not be immediately available in stand-alone primary percutaneous coronary intervention (PCI) centres.
Several observational studies have indicated that direct patient transfer to a cardiac centre may be of benefit in terms of survival and survival with good neurological recovery.13,29–33 However, as with studies evaluating the benefits of early angiography, these retrospective studies are at risk of selection bias. An important potential advantage of direct transfer is access to facilities that can lead to earlier decision making, including expert clinical diagnostics with performance of echocardiography followed by angiography and revascularisation where appropriate.
In cases of cardiogenic shock, where time-critical services such as coronary revascularisation and implantation of mechanical circulatory support devices may be of benefit, direct conveyance to centres with appropriate levels of care may be particularly pertinent.34,35 Although early angiography can lead to delays in delivery of TTM as observed in the Coronary Angiography After Cardiac Arrest (COACT) study, earlier stratification of patients at high risk of neurological injury could also provide an opportunity to focus early provision of TTM.36
A major disadvantage of direct conveyance to a specialist cardiac arrest centre is the financial and intensive burden in both acute and more long-term settings. It is known that the average OHCA patient has a hospital cost of £20,000, whereas costs for patients with a moderate to severe neurological disability (Cerebral Performance Category [CPC] 3–4) are significantly higher at £53,000.37 In the absence of clear benefit of the direct transfer of patients to a cardiac arrest centre, these costs may be difficult to justify. This question is currently being addressed by A Randomised tRial of Expedited transfer to a cardiac arrest centre for non-ST elevation out of hospital cardiac arrest (ARREST), which will randomise approximately 900 patients with OHCA without ST elevation to conveyance either to an emergency department or directly to the cardiac catheterisation laboratory.38
Targeted Temperature Management in OHCA
It is well established that interruption of cerebral blood perfusion after a cardiac arrest followed by reperfusion results in a cascade of events resulting in neuronal cell death. These events include astroglial phagocytosis, free radical generation and mitochondrial dysfunction.22 Reducing a patient’s temperature by 1°C decelerates cellular metabolism by 6–7%, and so artificial induction of ‘therapeutic hypothermia’ could provide protection from this deleterious process.39 Animal studies of therapeutic hypothermia in cardiac arrest have suggested that therapeutic hypothermia can be beneficial, but also that early delivery is mandatory to achieve benefit, with even a 15-minute delay after reperfusion injury resulting in more lesions in histopathological studies.40
Two landmark human trials initially suggested that induction of hypothermia could be beneficial. A study by the Hypothermia After Cardiac Arrest Study Group randomised 136 patients to 32–34°C or normothermia and found improved neurological outcome at 6 months in the hypothermia compared with normothermia group.41 A smaller study randomised 77 patients to 33°C or normothermia and also found improved functional outcome in the hypothermia group (49% versus 29%).42 This approach was extended to patients with a non-VF initial rhythm (in a non-randomised manner) who also appeared to acquire benefit.43 TTM was incorporated into ILCOR guidelines on the basis of these results, but questions remained about whether pyrexia in the normothermia group may have driven poor outcomes in this group.44
The seminal TTM trial aimed to answer these questions by randomising patients to moderate hypothermia (33°C) or mild hypothermia (36°C), and surprisingly found no differences between the two groups in the primary outcome of all-cause mortality or in the secondary outcome of a composite of poor neurologic function or death at 180 days.45 There has been much discussion about the unexpected findings of this trial, focusing on late commencement of TTM (~4 hours), late reaching of target temperature (~10 hours) and rapid rates of rewarming. However, hypothermia is not without its own systemic consequences, including coagulopathy, electrolyte imbalance, haemodynamic changes and altered drug pharmacokinetics.46,47
Due in part to the results of the TTM trial and perceptions of risk from hypothermia, current practice is to aim for 36°C in most cases. The Targeted Hypothermia Versus Targeted Normothermia After Out-of-hospital Cardiac Arrest (TTM-2) trial aims to address whether avoidance of pyrexia alone is important by randomising 1,900 patients to hypothermia at 33°C versus ≤37.8°C (NCT02908308).
On the theoretical basis that early initiation of hypothermia is critical to success, with a reported 20% increase in mortality with every hour of delay, intravascular infusion of cold saline has been used in both prehospital and centre settings.48 This holds particular promise in the cardiac catheterisation laboratory, where achievement of rapid hypothermia through conventional means, such as ice packs, can be challenging and lead to significant delays. Although rapid hypothermia can indeed be achieved by this method, this is at the cost of higher rates of re-arrest, pulmonary oedema and diuretic use, as well as lower rates of ROSC.49,50
Management in the Cardiac Catheterisation Laboratory
Emergency Angiography and Revascularisation in Primary Cardiac OHCA
There are numerous causes of OHCA, but a primary cardiac aetiology is common, either from underlying coronary artery disease (CAD) or myocardial disease. In post-mortem studies, 79.3% of young patients (mean age 38 years) with sudden cardiac death had a cardiac cause, with 56.7% of the total due to an acute MI; this figure was high as 73.3% in an older, unselected population.51,52 In clinical practice, there are similar rates of significant CAD as in these post-mortem studies. Spaulding et al. reported very high rates of acute coronary occlusion in a cohort of consecutive patients presenting with OHCA (48%).53 Since then, there have been several retrospective cohort studies that have found rates of CAD between 50% and 80%, although these studies generally included highly selected populations.25,27,54
One study that systematically evaluated the presence of CAD in a consecutive group of 257 patients with OHCA found rates of obstructive CAD (diameter stenosis >50%) of 63% in patients with ST elevation, 52% in patients with left bundle branch block (LBBB), 54% in patients with ST depression and 31% in patients with no acute changes.55 Rates of culprit lesions have been reported to be as high as 90% in patients with ST-elevation MI (STEMI), but culprit lesions are seen in 25–58% of patients even in the absence of ST-elevation on post-ROSC ECG.27,56,57 However, the high rates of obstructive CAD observed in this patient group do not provide a clear causal link to the cardiac arrest event, especially in the absence of a plaque rupture or the presence of a thrombus.
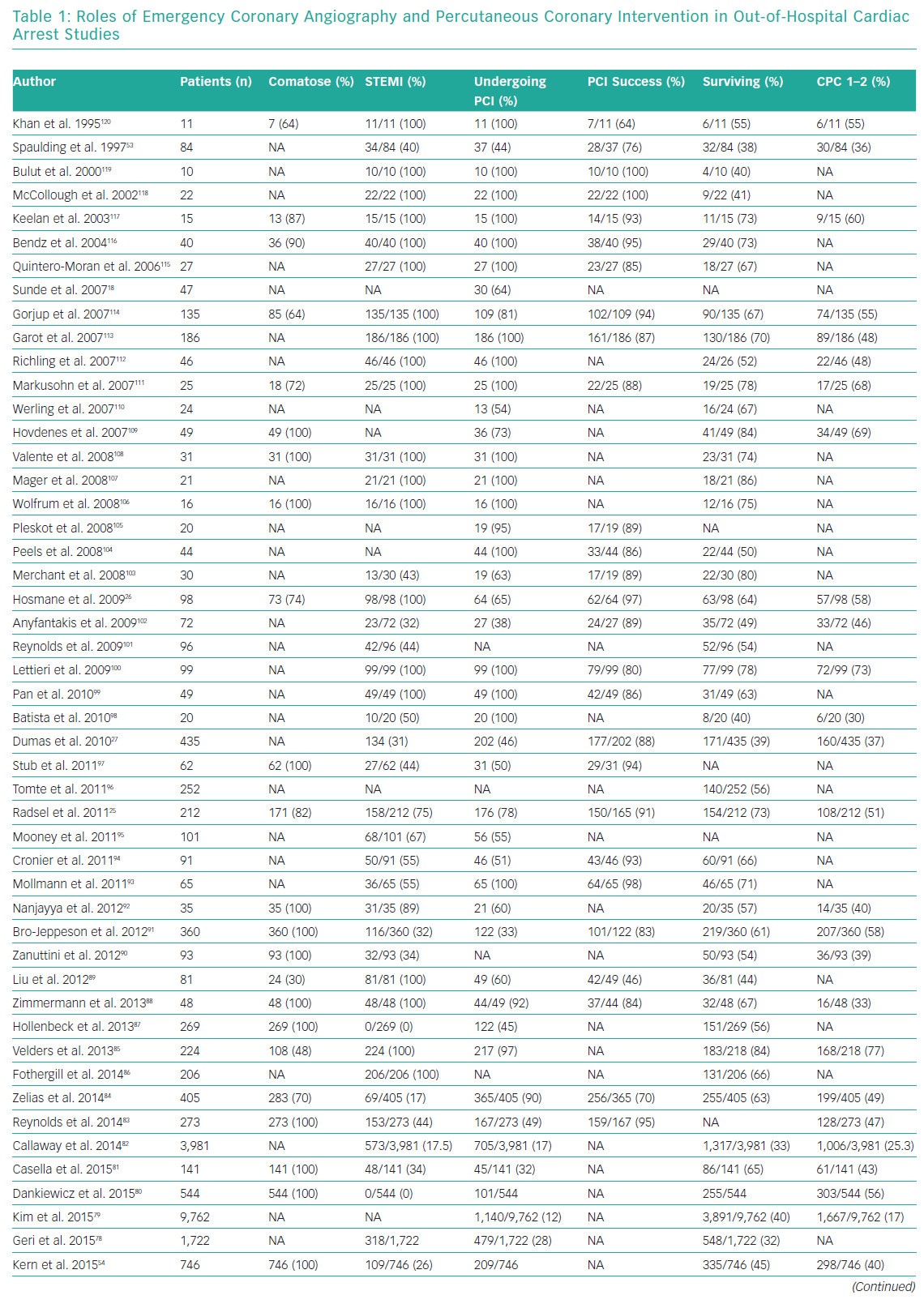
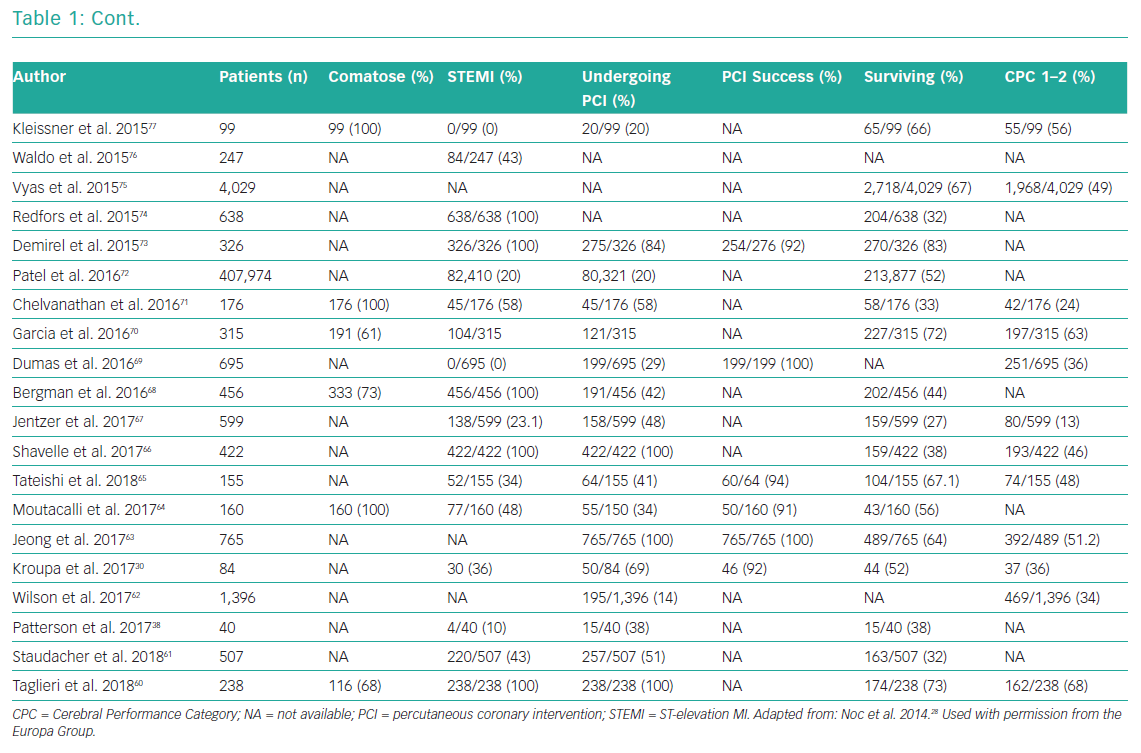
Whether emergency coronary angiography and subsequent PCI are of benefit following OHCA remains unclear, primarily because of a lack of data from randomised control trials (RCTs). OHCA patients have been systematically excluded from almost all clinical trials investigating revascularisation in an acute setting. Nonetheless, for patients with ST elevation or new LBBB at the time of ROSC, both European Society Guidelines (ESC) and American College of Cardiology Foundation/American Heart Association (AHA) guidelines recommend early coronary angiography (Class I, level b recommendation).58,59 Several observational studies and registries have evaluated the role of early angiography in patients with and without STEMI (Table 1).25,26,30,38,53,54,60–120
These studies confirm an increasing uptake of early coronary angiography, the feasibility and safety of its delivery and an indication that it may improve both survival and neurological recovery. The most contemporary meta-analysis included 15 observational studies and showed that survival was improved in the coronary angiography group compared with the group receiving conservative management (58.8% versus 30.9%; OR 2.77, 95% CI [2.06–3.72]), with a similar result for neurological outcome (58% versus 35.8%; OR 2.20, 95% CI [1.46–3.32]).121 However, these studies are observational and include highly heterogeneous populations, so these findings are susceptible to selection bias.
As with ST elevation, patients without ST elevation and OHCA have not been routinely recruited into RCTs. This group is especially heterogeneous and can include patients ranging from those with a normal ECG to those with profound ST depression. For this reason, both the ESC guidelines and the EAPCI recommend the provision of coronary angiography in patients without ST elevation if there is a high suspicion of a cardiac cause (i.e. chest pain before arrest, history of CAD and abnormal or uncertain ECG results) and in the absence of non-favourable arrest circumstances.28,122
A recently reported meta-analysis that included seven observational studies and one RCT and >2,000 patients suggested that early coronary angiography may improve survival compared with delayed or no angiography (19.6% versus 35.6%; p<0.001).123 However, these studies are also observational, and this question is currently the subject of several on-going RCTs, including the Pilot RCT of Early Coronary Angiography Versus Delayed Coronary Angiography (PEARL; NCT02387398), Immediate Unselected Coronary Angiography Versus Delayed Triage in Survivors of Out-of-hospital Cardiac Arrest Without ST-segment Elevation (TOMAHAWK; NCT02750462), EMERGEncy Versus Delayed Coronary Angiogram in Survivors of Out-of-hospital Cardiac Arrest (EMERGE; NCT02876458) and Direct or Subacute Coronary Angiography in Out-of-hospital Cardiac Arrest (DISCO; NCT02309151).
The COACT study was the first to recruit patients with OHCA without ST elevation into a randomised clinical trial comparing early versus delayed angiography.124 The overall finding of the study was that there was no benefit from early angiography in terms of mortality at 90 days. It is important to note that patients with shock and estimated glomerular filtration rate <30ml/min/1.73m2 were excluded from that study and, perhaps as a result, there was a lower than expected rate of culprit lesions (13%) and higher survival rate in both arms (65%). Nonetheless, most patients died from severe neurological injury regardless of randomisation arm.125
It is plausible that provision of early PCI in the presence of an acute thrombotic occlusion or multiple obstructive lesions may limit the extent of cardiogenic shock, leading to improvements in left ventricular function and potentially protection from hypoxic brain injury. The benefits of restoration of coronary flow in this setting have been indicated in large animal studies.126 Current guidelines recommend emergency PCI for an acute occlusion, the presence of thrombus or abnormal flow, and these findings should, where appropriate, be correlated with ECG and echocardiography.28 The relative effects of acute plaque rupture and associated ischaemia in the pathophysiology of OHCA remain unclear. Intracoronary imaging, particularly optical coherence tomography, may be of some use in illustrating these processes by delineating plaque morphology and identifying plaque rupture and thrombus, which may guide decision making, although the evidence for intracoronary imaging use in the OHCA population is limited.127
Multivessel disease is common in the OHCA population (in up two-thirds of patients) and is associated with higher rates of cardiogenic shock, which itself is observed in approximately 50% of patients with OHCA.128,129 The role of multivessel PCI in the OHCA population has primarily been studied in the presence of cardiogenic shock, which is usually defined as a systolic blood pressure <90 mmHg or the requirement for ionotropic therapy to maintain this blood pressure. In the non-OHCA STEMI population, there have been several recent landmark trials that have suggested that multivessel PCI during the initial admission is safe and may provide clinical benefit.56,130–132 Results of studies evaluating the role of multivessel PCI in the OHCA population are conflicting, particularly in those patients with cardiogenic shock. Systematic reviews of observational studies have suggested that this approach could be beneficial, although noting inherent selection bias.133,134
The SHould we emergently revascularize Occluded Coronaries in cardiogenic shocK (SHOCK) trial, in which 30% of patients had an OHCA, showed that early coronary revascularisation improved survival at 1 year compared with initial medical stabilisation (46.7% versus 33.6%).135 That study was not reflective of contemporary practice, with high rates of thrombolysis, 80% of patients receiving culprit PCI only and stent placement in only 35.4% of patients.
The Culprit Lesion Only PCI Versus Multivessel PCI in Cardiogenic Shock (CULPRIT-SHOCK) trial was a large, contemporary study that included a large proportion of patients following OHCA. In that study, despite improvements in care, advances in PCI techniques and stent technologies, there was limited improvement in mortality (43.3% across all patients) compared with the SHOCK trial.136 Culprit vessel PCI was superior to multivessel intervention alone in the primary endpoint of death or renal replacement therapy at 30 days (45.9% versus 55.4%, respectively).136 At 1 year, there was no difference in mortality between the two arms (50% versus 56.9% for culprit vessel versus multivessel PCI, respectively).137 As with the SHOCK trial, approximately 50% of patients recruited into the CULPRIT-SHOCK trial also had sustained OHCA, suggesting that this strategy may be appropriate in the OHCA population. In practice, a pragmatic approach should be adopted where multivessel PCI is restricted to those exhibiting poor clinical and haemodynamic responses to treatment of the culprit lesion.
Should the Patient be Taken Directly to the Cardiac Catheterisation Laboratory?
Decisions to take patients to the cardiac catheterisation laboratory are complex and are currently based on pragmatic assumptions of the likelihood of a culprit lesion, futility and the presence of haemodynamic instability, but with limited supportive clinical evidence. In contrast with previous observational trials in which patients with OHCA were selected for angiography on the basis of clinical discretion, there was a surprisingly low rate of culprit CAD in the COACT randomised clinical trial (i.e. in 13% of patients, with acute atherothrombotic lesions only in 3% of patients).125 Hence, methods of improving prediction of a culprit lesion are urgently required to guide an invasive strategy.
The ACS2 score incorporates clinical features of cardiac arrest (presence of angina and congestive heart failure on admission), initial rhythm and 12-lead ECG findings in order to define the presence of a culprit lesion. In patients with STEMI, the area under the curve (AUC) was 0.88, but the discriminant ability of the ACS2 score was significantly lower in patients without ST elevation (AUC = 0.74).76 Objective prediction of futility on arrival to the cardiac catheterisation laboratory is another important consideration but currently remains a significant challenge. Risk algorithms and biomarkers hold significant potential in this regard, but these have currently failed to translate to the clinical realm, partly because they have had limited application and validation on arrival, when emergency treatment decisions are made.138
The Cardiac Arrest Hospital Prognosis (CAHP) score is a multivariable nomogram that is used on arrival to the intensive treatment unit (ITU) and predicts poor neurological recovery on ITU discharge.139 A subanalysis of that study indicated that patients with lower predicted risk of neurological injury benefit more from an early invasive approach than those at higher risk.140 This has been replicated in other studies, where patients with the most severe form of cardiac arrest were most likely to die from neurological causes.83,141 Finally, it is accepted that patients with on-going haemodynamic instability may benefit more from revascularisation, as indicated by the results of the SHOCK study.142 However, whether patients after OHCA with cardiogenic shock may additionally benefit from early implantation of mechanical circulatory support devices remains unclear. The CREST model identified five variables in patients without ST elevation that could predict, with moderate accuracy, patients at higher risk of cardiac aetiology death.143 Integration of these risk scores into prospective clinical trials may provide objective information to support decision making on arrival to the cardiac catheterisation laboratory.
Pharmacological Considerations
Pharmacological treatment in conscious survivors of OHCA does not differ from management of patients with acute coronary syndrome without cardiac arrest.57 However, comatose survivors of OHCA pose a unique challenge, mostly because they are mechanically ventilated and thus unable to take drugs orally. This can often lead to significant delays in the administration of many drugs until a gastric tube is inserted. In addition, several other factors may play a role in the efficacy of antiplatelet drugs, including reduced intestinal drug absorption due to gastroparesis and hypoperfusion, therapeutic hypothermia and increased platelet reactivity in response to systemic inflammation following resuscitation.123 These mechanisms place OHCA patients at a particularly high risk of acute and subacute stent thrombosis, ranging from 1.4% up to 31%.144-148 In contrast, as a result of possible injuries due to chest compression, intubation and trauma during the OHCA, these patients are also at an increased risk of bleeding.149
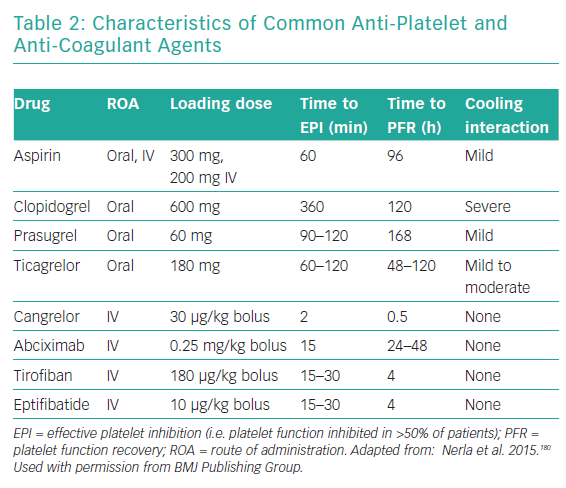
Antiplatelet drugs represent the fundamental pharmacological treatment in patients receiving coronary stents. The pharmacological characteristics of the commonly used anti-platelet drugs used are summarised in Table 2. Llitjos et al. reported that 45% of comatose OHCA survivors had an insufficient response to aspirin regardless of the route of administration, possibly due to increased platelet reactivity in the setting of the OHCA.150 Nevertheless, closure time, which is a measure of platelet inhibition in vitro, was significantly increased in the intravenous group, suggesting that a parenteral route of administration is the preferred choice.150 Moreover, the intravenous route is justified not only for initial, but also for subsequent aspirin administration because the absorption of enterally administered aspirin remains affected by hypothermia.151
Oral inhibitors of the ADP P2Y12 receptor, which can be given only via a nasogastric tube, have delayed onset of antiplatelet activity, particularly in the case of clopidogrel.152 Even in the case of novel and more potent agents, such as ticagrelor, there is still a 3- to 4-hour delay until target platelet inhibition is reached.153 The antiplatelet effects of all oral P2Y12 receptor inhibitors, including ticagrelor, are known to be reduced by therapeutic hypothermia, which, in turn, leads to a higher rate of stent thrombosis.154 Prüller et al. compared platelet inhibition in patients in whom the new intravenous P2Y12 inhibitor cangrelor was used to bridge the gap observed with the oral P2Y12 inhibitors and observed a significantly higher anti-aggregation effect with cangrelor after 8 hours without an excess of bleeding.155
Further prospective studies are needed to specifically investigate whether periprocedural treatment with cangrelor can bridge a 3-hour gap in platelet inhibition following administration of the novel oral P2Y12 inhibitors via nasogastric tube in comatose survivors of OHCA. Conversely, the clinical benefits of ticagrelor over clopidogrel in this high-risk subset of patients remain to be robustly proven. A recent meta-analysis showed that there was no difference in the incidence of stent thrombosis (6.1% versus 6.3%), in-hospital mortality or major bleeding between clopidogrel and the newer P2Y12 inhibitors ticagrelor and prasugrel.156
There have been no specific studies regarding anticoagulation in comatose survivors of OHCA undergoing PCI, and currently unfractionated heparin is generally used. Glycoprotein IIb/IIIa inhibitors are used as a bailout strategy at the discretion of the interventional cardiologist, who also has to consider the increased risk of bleeding in these patients.58 In a recent observational study of 71 patients with OHCA treated with therapeutic hypothermia, the use of glycoprotein IIb/IIIa inhibitors was associated with increased bleeding risk with no benefit with regard to thrombotic events.157 Owing to a lack of appropriately sized prospective clinical trials in this area, most studies in this field to date have been insufficiently powered to enable satisfactory conclusions to be drawn.
Mechanical Circulatory Ventricular Support in OHCA-associated Cardiogenic Shock
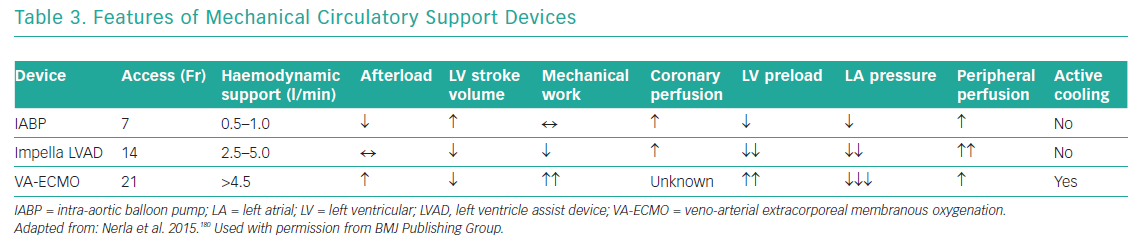
Mechanical circulatory ventricular support devices are potentially useful in the OHCA population with cardiogenic shock, present in approximately half of all patients.128 The putative benefits of such devices are attenuation of cardiogenic shock, which is the cause of death in one-quarter of patients, temporary relief of myocardial dysfunction (‘stunning’) and minimisation of multiorgan dysfunction (Table 3).16
The use of supportive pharmacotherapy is near ubiquitous in cardiogenic shock despite limited evidence supporting its use. The use of catecholamines, such as noradrenaline, adrenaline and dobutamine, improves myocardial contractility and leads to systemic and arterial vasoconstriction. Although this causes a temporary improvement in haemodynamics, it can paradoxically lead to an increase in cardiac work, oxygen consumption and disproportionate arterial vasoconstriction, all of which can have systemic deleterious effects.158 In two randomised controlled trials, noradrenaline had the same effect on cardiac index as adrenaline but with less deleterious effects on heart rate and lactate.159 This led to lower rates of refractory cardiogenic shock in those treated with noradrenaline then adrenaline (37% versus 7%; p=0.008).159 Dobutamine is a powerful inotrope but with a relative vasodilatory effect compared with the other catecholamines owing to increased beta-2 action, which may be of particular benefit in patients with cardiogenic shock who exhibit profound systemic vasoconstriction. Dopamine has traditionally been used in cardiogenic shock, but in a large trial of patients with cardiogenic shock its use was associated with higher rates of adverse events than in patients treated with noradrenaline.160
Nonetheless, current ESC guidelines recommend the use of noradrenaline as a first-line agent with Class IIb, level of evidence B.161,162 Novel agents, such as levosimendan (a calcium sensitiser) and milrinone (a phosphodiesterase inhibitor), have the potential to improve contractility without significantly increasing metabolic requirements whilst inducing an element of vasodilatation. However, there is extremely limited randomised trial data for their use, and studies are required to understand their contemporary use in cardiogenic shock.163
Several devices are currently available, including intra-aortic balloon pumps (IABP), the Impella family of left ventricular assist devices (LVADs; Abiomed) and devices to provide veno-arterial extracorporeal membranous oxygenation (VA-ECMO).
Intra-aortic Balloon Pumps
IABPs are counterpulsation devices situated in the descending aorta that inflate in diastole and deflate in systole. This reduces cardiac afterload and increases coronary blood flow, resulting in a small increase in cardiac output (0.5–1 l/min), lowers wall stress and reduces myocardial oxygen consumption, although with a modest reduction in the mechanical work of the heart.164 An IABP was used in 86.1% of patients in the SHOCK trial, and equally in the revascularisation and initial medical stabilisation groups.135 The role of IABPs in cardiogenic shock was specifically studied in the IABP SHOCK II trial, an RCT that recruited 600 patients, nearly half of whom had suffered cardiac arrest. In that study, there was no difference in the primary endpoint of mortality or several secondary endpoints, such as bleeding, sepsis and lactate levels, between the IABP and control groups.165 The timing of implantation before or after PCI had no effect on outcome.166 On the basis of these results, both ESC and AHA guidelines no longer recommend routine use of IABPs in cardiogenic shock.58,59,122
Impella Left Ventricular Assist Devices
The Impella is a percutaneous mini-axial flow LVAD that is placed across the aortic valve and has three different platforms that generate 2.5–5 l/min cardiac output. The Impella LVAD reduces preload and the mechanical work of the heart, and improves haemodynamic parameters.158 The Efficacy Study of LV Assist Device to Treat Patients With Cardiogenic Shock (ISAR-SHOCK) trial compared IABPs and the Impella 2.5 in 26 patients with cardiogenic shock.167 The Impella 2.5 did not significantly improve the cardiac index (from 1.71 l/min/m2 at baseline to 2.20 l/min/m2) compared with the IABP (from 1.73 l/min/m2 at baseline to 1.81 l/min/m2).167 That study was underpowered and did not show any difference in mortality at 30 days. Furthermore, the Impella failed to show benefit compared with the IABP in the Initial Management of Patients Receiving a Single Shock (IMPRESS) trial, which was a small trial that recruited 49 patients randomised against the IABP.168 Again, half the patients recruited into that trial had suffered a cardiac arrest, and there was a high rate of neurological injury leading to death. As with ISAR-SHOCK, the IMPRESS trial was significantly underpowered for clinical endpoints. A meta-analysis including 2,483 patients from 13 trials comparing IABPs, the Impella LVAD and conservative therapy in patients with cardiogenic shock found that all strategies had equal outcomes, but that Impella use was associated with higher bleeding rates.169
A retrospective study of 287 patients found that early implantation of Impella (prior to PCI) in cardiogenic shock was associated with improved survival (66% when mechanical circulatory support was initiated <1.25 hours from shock onset, 37% when initiated within 1.25–4.25 hours, and 26% when initiated after 4.25 hours; p=0.017).34 In the CULPRIT-SHOCK trial, there were relatively high rates of mechanical circulatory support device implantation, where it was used in 27.4% of patients, of which 42.2% were implanted with the Impella.137 Results from a small consecutive series indicate that Impella use may be of benefit at the time of refractory VF OHCA with on-going CPR.170 In that study, survival with good neurological function was recorded in 50% of patients, but the major vascular complication rate was 50%.170
Extracorporeal Membranous Oxygenation
VA-ECMO is a mode of circulatory bypass that can support both ventilatory and circulatory function and can be used in the cardiac catheterisation laboratory but is currently available primarily in supraspecialist units.142 VA-ECMO returns oxygenated blood at high flow rates to the arterial system, achieving a cardiac output of 2.5–5 l/min with a reduction in preload and improvement in systemic tissue perfusion. However, because blood is often returned into the descending aorta, there is an increase in afterload with incomplete unloading of the left ventricle (LV),171 which reduces LV stroke volume and, in turn, increases LV wall stress and mechanical work of the heart and potentially reduces coronary perfusion.172 Concurrent use of the IABP may enable improved coronary perfusion in diastole, reduced wall stress and improved stroke volume, whereas the Impella device has higher flow rates with more effective unloading of the LV.173
Miniaturisation of ECMO equipment and percutaneous cannulation has made its use increasingly feasible, leading to increased use worldwide.174,175 However, the benefit of ECMO in cardiogenic shock is uncertain owing to a lack of RCTs. Several non-randomised observational studies were included in a pooled analysis of 1,116 patients with cardiogenic shock (of whom 540 had cardiac arrest), with the results suggesting that survival to hospital discharge is in the region of 40% with a better outcome in the cardiogenic shock group than in those following OHCA, possibly because of a higher rate of ‘neurological death’ in the latter group (52.5% versus 36.2%).176 The complication rate with ECMO in that study was high, with 47.4% of patients developing renal impairment, 25% developing infection and 13.1% developing persisting neurological deficits. Although these studies cumulatively include large populations and reflect real-world practice, they are a combination of several small observational studies and have significant selection bias; therefore, the results need to be interpreted with caution.
ECMO can also be used in extracorporeal CPR (ECPR), where mechanical circulatory support is used to augment cardiopulmonary function in cases of refractory cardiac arrest, defined as a failure to respond to conventional CPR. ECPR can be used on admission to a cardiac arrest centre or at the scene. Data from 295 patients in the Extracorporeal Life Support Organization (ELSO) registry found a survival to discharge after ECPR of 27%,177 and a propensity matched study found that use of ECPR was an independent predictor of survival to discharge (HR 0.51, 95% CI [0.35–0.74], p<0.0001) and at 1 year (HR 0.53, 95% CI [0.33–0.83], p=0.006).178
A meta-analysis from 2017 that included six studies and in which 376 patients received ECPR demonstrated an improved survival to discharge compared with conventional treatment (relative risk [RR] 2.37, 95% CI [1.63–3.45], p<0.001) and better long-term neurological outcome (RR 2.79, 95% CI [1.96–3.97], p<0.001).179 However, these findings should be seen as hypothesis generating because they involve highly selected cases. A Comparative Study Between a Pre-hospital and an In-hospital Circulatory Support Strategy (ECMO) in Refractory Cardiac Arrest (APACAR2) (NCT0252703), which is currently recruiting, will evaluate prehospital ECPR versus in-hospital ECMO and provide further insights.
At present, the use of mechanical circulatory support devices, particularly Impella and ECMO, cannot be recommended in patients with OHCA or in cardiogenic shock on the basis of current evidence. Identification and risk stratification of patients who may benefit the most from these highly invasive and costly therapies is urgently required. Ongoing controlled trials, such as Testing the Value of Novel Strategy and Its Cost Efficacy in Order to Improve the Poor Outcomes in Cardiogenic Shock (EUROSHOCK; NCT03813134) and Early Initiation of Extracorporeal Life Support in Refractory OHCA (INCEPTION; NCT03101787), may help address the current uncertainties.
Future Directions
Urgent clinical trials and translational research to address numerous uncertainties in this field are required. Firstly, the ECG is currently used as the frontline tool for decision making, but this is known to be a poor identifier of CAD in this group of patients, and novel assessment tools or biomarkers may, in future, allow for improved discrimination. Secondly, the effects of hypoxic brain injury overwhelm the potential benefit of PCI in all-comers with OHCA because this remains the leading cause of death. Risk stratification tools and biomarkers validated on arrival to a cardiac centre to identify those at high risk of hypoxic brain injury may enable a more nuanced decision-making process. Thirdly, the role of mechanical circulatory support devices in this population remains unclear. A better understanding of the relationship between haemodynamic and metabolic phases of shock, together with improved patient stratification, will improve the optimal harnessing of these novel technologies. Finally, in order for tangible improvements in patient outcomes to be realised, an improved evidence base from translational research and well-conducted clinical trials is required to facilitate appropriate patient selection for aggressive invasive and supportive cardiovascular interventions.
Conclusion
OHCA remains an important cause of death in developed countries and there is a significant drive to improve early and long-term outcomes. It is common for OHCA to have primary cardiac cause, so pathways of care that convey patients directly to a specialist cardiac centre may be advantageous, and this has been recognised in recent guidelines.22–24 However, bringing all patients with OHCA directly to a cardiac centre will place a significant resource burden on these units with, as yet, limited evidence that outcomes are improved in an unselected population. Observational data suggest that direct conveyance of the Utstein comparator cohort to heart attack centres is beneficial and may set a paradigm for extension to the undifferentiated population.