Dr. Basir is an Interventional Fellow at the Henry Ford Hospital in Detroit, Michigan.
Dr Basir commenced his presentation by reminding the audience that, despite the fact that the number of cases of cardiac shock (CS) during acute myocardial infarction (AMI) has steadily risen,1 the rates of in-hospital mortality have remained unchanged for more than 20 years.2 A group of physicians, including Dr Basir, examined the Abiomed Impella Quality (IQ) database on Impella use, with the aim of identifying factors that may be associated with survival. They used this information to derive an institutional protocol that could be systemically implemented across several hospitals in the region of the Henry Ford Hospital. This prospective approach was focused on improving survival in this patient population. Of note, there is a wide variation in outcomes with Impella use across different sites: IQ data (791 sites supporting >4 patients with AMI CS, 15,529 patients total) show that the bottom 20 % performing sites have a mean survival of only 30 %, whereas the top 20 % of sites have a higher volume of Impella utilisation and a mean survival of 76 %. In 2016, the mean survival rate was 58 %, a relative improvement of 14 % since the US Food and Drug Administration (FDA) approval on the Impella.3
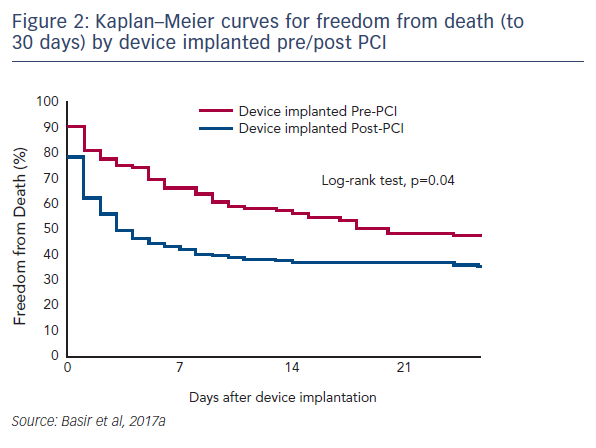
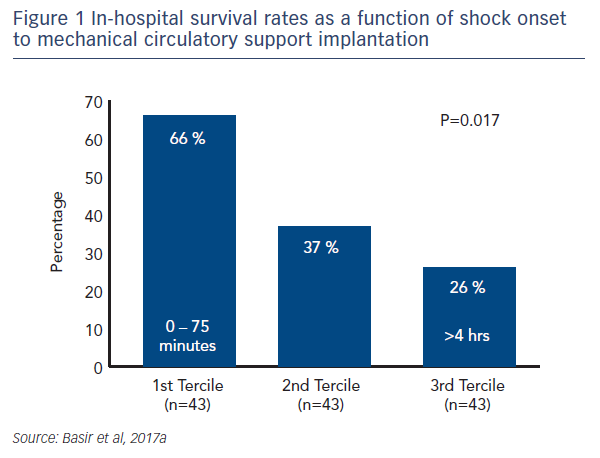
One factor observed to be associated with early mortality in AMI/CS is increased inotrope exposure.4 This does not determine causality as the severity of a patient’s condition correlates with the number of inotropes and vasopressors. Nevertheless, it is likely that the load of inotropes and vasopressors directly influences outcomes.5 Similarly, a delay in support is clearly associated with mortality in AMI/CS. Data indicate that if a patient receives mechanical circulatory support (MCS) in the first 75 minutes following AMI, outcomes are substantially improved compared with those who have a longer delay in support (see Figure 1).5 In addition, the use of haemodynamic support prior to percutaneous coronary intervention (PCI) has been shown to improve survival, due to effects on the reperfusion injury and ischaemia (see Dr Navin Kapur’s talk). The separation of the Kaplan–Meier curves occurs very early following PCI, reinforcing the idea that early MCS is a key determinant in clinical outcomes (see Figure 2).5
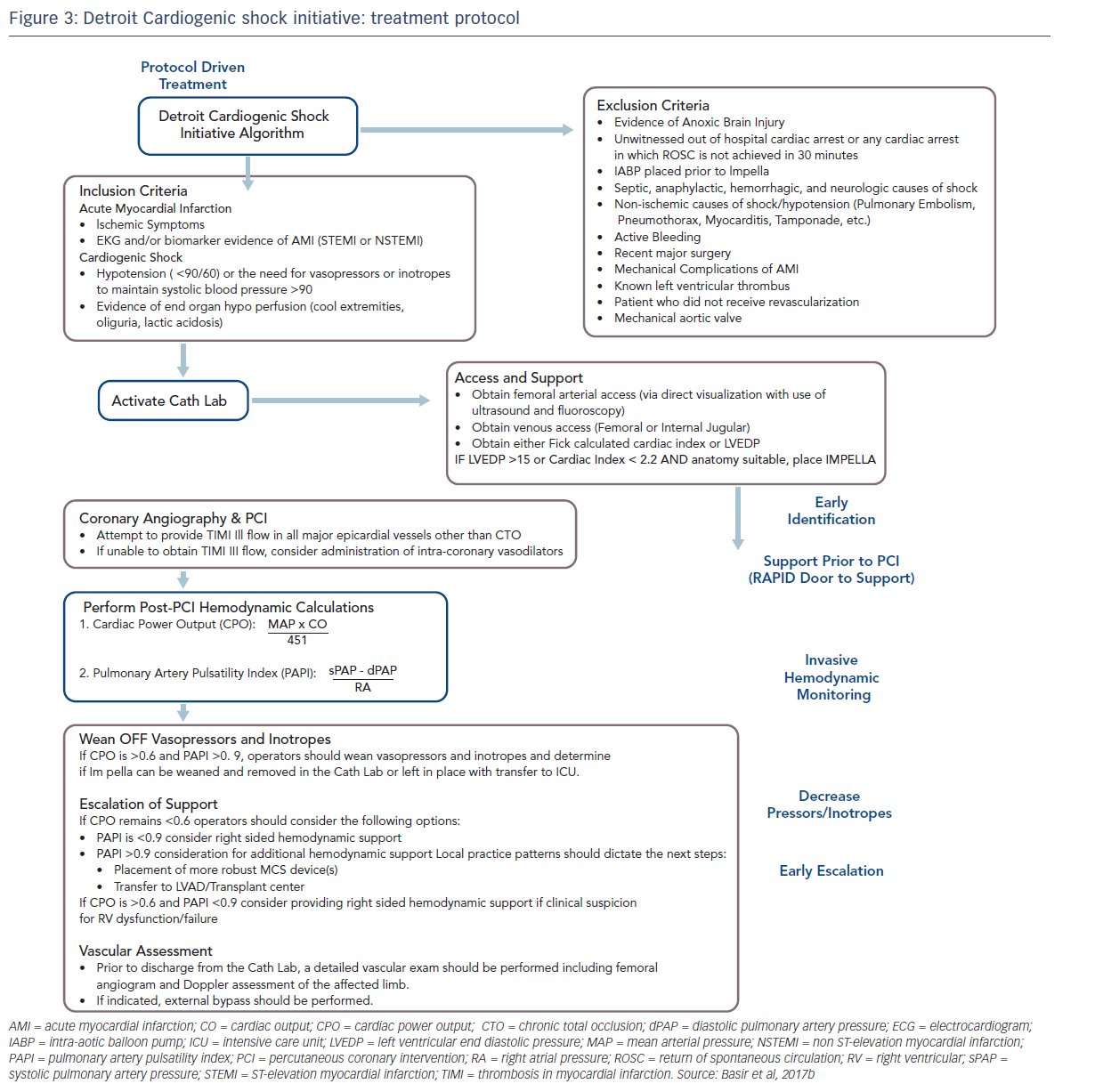
These factors have been used to develop a protocol for use by the Detroit Cardiogenic Shock Initiative (CSI), a collaboration between four hospitals in Detroit, under the leadership of Dr William O’Neill, with the aim of increasing survival in MI/CS (see Figure 3).6 This protocol is specific to a defined group of patients, and has proscribed exclusion and inclusion criteria. Although this was a protocol-led treatment, individual decisions were based on operator preference. Nevertheless, this approach allows for better assessment of real-world outcomes. The protocol was comprised of early detection of CS, immediate catheterisation laboratory activation, mechanical support prior to PCI, invasive haemodynamic monitoring, decreased vasopressor/inotrope use and early escalation to a larger support device if needed (cardiac power output < 0.6 W and a pulmonary artery pulsatility index <0.9). Quality measures include doorto- support time of less than 90 minutes, establishment of Thrombolysis In Myocardial Infarction (TIMI) III flow, weaning of vasopressors and inotropes, maintaining a cardiac power output in excess of 0.6 W and improving survival to discharge.
Using this protocol, the Detroit CSI pilot study has been initiated and has treated 41 patients at the time of the A-CURE Symposium. The average age of participants was 65 years, and 70 % were male. A total of 95 % were taking vasopressors and 41 % were in cardiac arrest.6 This patient populations is similar to that of the SHOCK trial (n=302).7 The population differed from that in the Impella versus Intra-Aortic Balloon Pump in Cardiogenic Shock (IMPRESS) trial (n=48), a prospective trial in which the patients were much sicker, all were mechanically ventilated and 92 % had a cardiac arrest.8 In the Detroit CSI study, the median lactate levels were 4.7 g/dl compared with 8.2 g/dl in the IMPRESS trial.
Of 55 screened patients, 14 were excluded based on the inclusion/ exclusion criteria. The pilot study showed favourable outcomes. Outof- hospital cardiac arrest occurred in 6 participants and there were 11 in-hospital cardiac arrests. Overall survival rate was 76 %, compared with 53 % in the SHOCK trial and 53 % in IMPRESS.7,8 Implantation of Impella prior to PCI occurred in 66 % of participants and there was a 66 % improvement in cardiac power output (0.57 W to 0.95 %; P<0.001) after the initiation of MCS and PCI. Of note, as the study progressed, protocol adherence increased, with a corresponding improvement in outcomes.6
In conclusion, rapid early delivery of MCS guided by invasive haemodynamic monitoring is associated with significantly improved survival in an AMI/CS patient population. This multi-institutional effort demonstrates the effectiveness of an institutionalised protocol to address CS and significantly improve patient outcomes in this difficult patient cohort. Dr Basir described this as a “war on shock”, which involves a systematic team effort using regional shock protocols that can be summarised as follows:
A – Access
B – Basic Haemodynamics (blood pressure, left ventricular end diastolic pressure and cardiac power output)
C – Circulatory Support
D – Decrease vasopressors and inotropes
E – (Early) Escalation.