Although a combination of multiple strategies to prevent and treat coronary artery disease (CAD) has led to a relative reduction in cardiovascular mortality over recent decades, CAD remains the greatest cause of morbidity and mortality worldwide.1 Based on clinical presentation and prognosis, CAD spans from stable presentations (e.g. chronic angina pectoris) to acute coronary syndromes (ACS), which encompass a variety of clinical scenarios (e.g. unstable angina [UA], non-ST-elevation MI [NSTEMI] and ST-elevation myocardial infarction [STEMI]). Guideline-directed medical therapy and revascularisation using percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) are the main treatment strategies across the spectrum of CAD.2
A variety of individual factors and circumstances other than clinical presentation and treatment type contribute to determining the outcome of CAD. It is increasingly understood that personalised medicine that takes these factors into account achieves superior results than “one-size-fits-all” approaches, which may ignore, for example, individual risk of ischaemia or propensity to bleed with antithrombotics. Directing appropriate treatment strategies to the right individuals is a clinical challenge.
In making decisions when more than one treatment is available, physicians may rely entirely on their judgement, use clinical algorithms or be assisted by risk scores. Risk scores can be diagnostic or prognostic, and the latter is more difficult to develop and validate because of the stochastic and time-varying nature of clinical outcomes.3
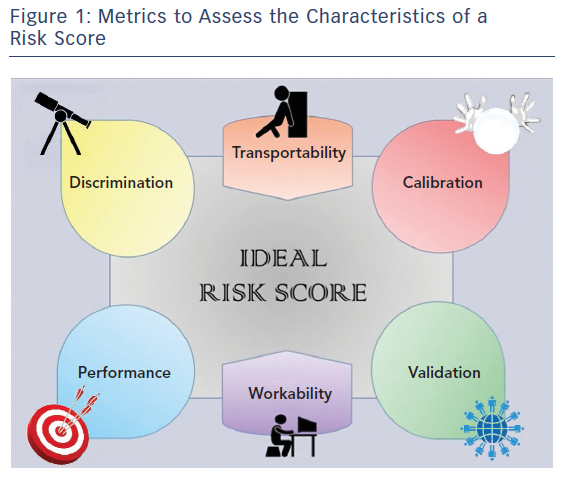
An ideal prognostic risk score should satisfy different characteristics (Figure 1). The ability to distinguish between high and low risk is called “discrimination”, and this is mathematically represented by a measure of concordance, the c-statistic (whose values range from 0.5 for worst discrimination and 1.0 for perfect discrimination).4 “Calibration”, which illustrates the similarity between predicted and observed risk, is identified by the Hosmer-Lemeshow goodness-of-fit test, with a chi-squared distribution in which the lower the value, the higher the calibration. The performance of a score, which concerns its accuracy in term of prediction, is measured by the Brier score, with values ranging from 0 (perfect prediction) to 1 (worst prediction). Finally, a risk score should prove to be effective (‘valid’) in similar but independent populations.
In recent years, the multiplication of risk scoring systems for CAD has generated some degree of uncertainty regarding whether, when and how predictive models should be adopted in driving clinical decisions. This article aims to review the most accepted prognostic risk models for patients with evidence of CAD.
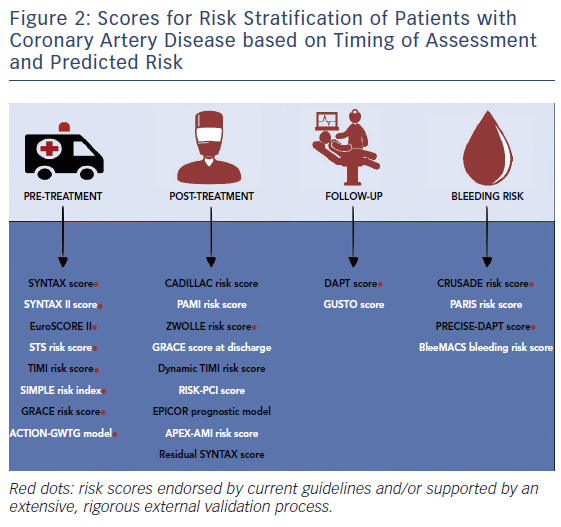
For the sake of clarity, risk scores have been grouped according to the following criteria: logical temporal sequence of CAD management (i.e. pre-treatment, treatment, post-treatment/discharge and follow-up); clinical presentation (stable CAD undergoing PCI versus ACS presentation); and outcome prognostication (i.e. prediction of ischaemic or bleeding risk) (Figure 2). Risk scores for ruling out the presence of CAD have been discussed in detail elsewhere and are beyond the scope of this article.6
Assessment of Risk Before Treatment
Stable Coronary Artery Disease
In patients with stable CAD, a shared multidisciplinary therapeutic decision-making process looking at the identification of the best treatment strategy – the local heart team discussion – is standard of care in clinical practice. In contrast with ACS management, where timely intervention is required and prognostically beneficial, stable CAD allows for a detailed diagnostic and therapeutic workup. Different risk scores can be useful aids to better inform the heart team discussion.
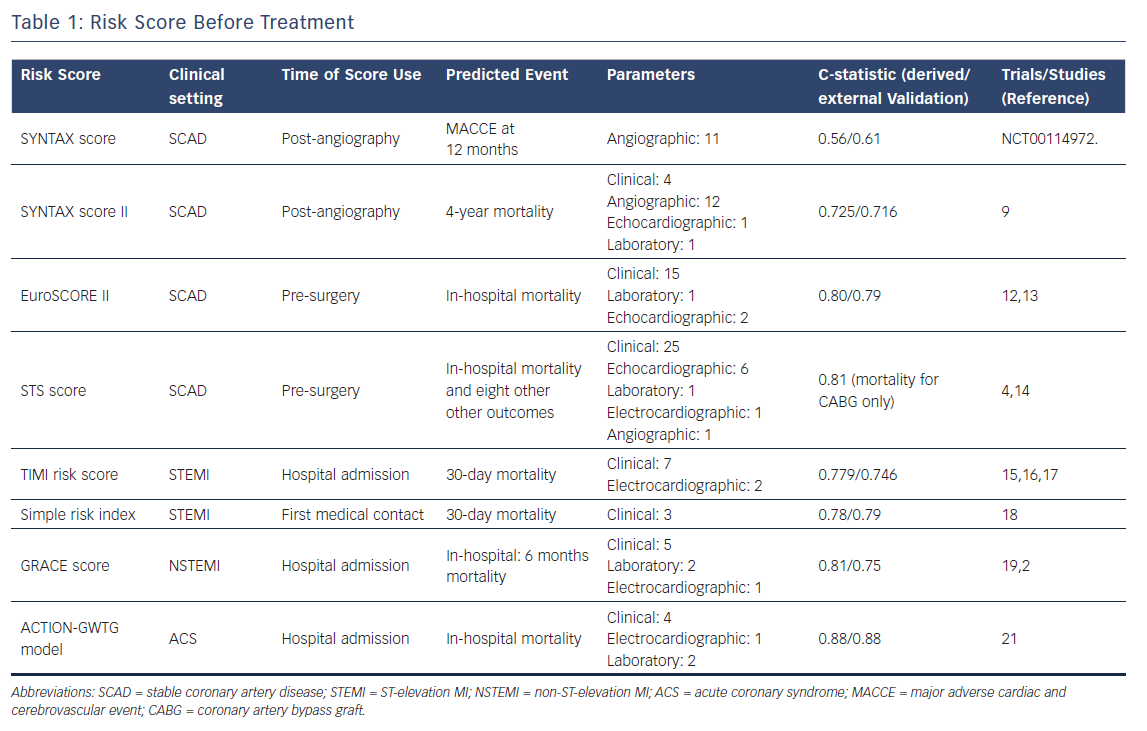
SYNTAX and SYNTAX II score
The SYNTAX score evaluates the anatomical burden and complexity of CAD by using a multi-parametric quantification tool based on coronary angiography (i.e. 12 questions ranging from anatomy to characteristics of lesion subsets such as bifurcations or chronic total occlusions). As endorsed by European guidelines, the SYNTAX score should be implemented as a tool to inform the decision-making processes between different revascularisation strategies (i.e. percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG).7
Based on the results of the landmark SYNTAX trial where a systematic evaluation of its score was used for the first time,8 SYNTAX scores of >22 and >32 should contraindicate (class III) revascularisation by PCI in patients with multivessel or left main disease, respectively.
However, the anatomical SYNTAX score has limitations, which result mainly from the variability in assessing and rating complex anatomies (leading to inter-observer variability) and the lack of clinical variables that might affect prognosis. To partly overcome these limitations, the SYNTAX II score has been developed with the aim of integrating the anatomical SYNTAX score with a small array of clinical variables (e.g. unprotected left main coronary artery disease, female sex, chronic obstructive pulmonary disease, age and left ventricular ejection fraction), which differently affect 4-year mortality in patients treated with PCI or CABG. By providing data on the expected mortality for each revascularisation strategy, the SYNTAX II score allows for individualised decision making between CABG and PCI. A user-friendly online calculator of the SYNTAX I and II score is available at www.syntaxscore.com.
In the recently published SYNTAX II study, the SYNTAX II score was used to select a cohort of patients with similar predicted risk of mortality for CABG and PCI treatment. In these patients, PCI performed according to a state-of-the-art treatment strategy (i.e. intravascular imaging guidance, functional evaluation of coronary stenosis by fractional flow reserve and optimised secondary prevention) had similar clinical outcomes at one year as a historical and equipoise-derived cohort of patients undergoing CABG in the SYNTAX trial.9
Functional SYNTAX score
Because the anatomical complexity of CAD is a major determinant of prognosis but additional factors contribute to prognosis, a series of iterations and refinements of the anatomical SYNTAX score have recently been proposed.
Beyond the above-mentioned SYNTAX II score, which clinically refines the anatomical SYNTAX score, the functional SYNTAX score evaluates the impact on the total ischaemic burden of different coronary stenoses by using information from fractional flow reserve (FFR) measurements. In the calculation of the functional SYNTAX score, only lesions leading to significant ischaemia are rated while non-functionally relevant stenoses are ignored. This process is used to refine the anatomical SYNTAX score with functional information.
The functional SYNTAX score outperformed the anatomical SYNTAX score in patients with multivessel disease enrolled in the FAME trial (n=497), with better discrimination for major cardiac adverse events (MACE) at one year (c-statistic of 0.677 versus 0.630 for functional versus anatomical SYNTAX score respectively; p=0.02).10
Advances in CT of coronary arteries have recently allowed functional SYNTAX score to be assessed without the need for invasive cardiac catheterisation.11 This approach might be useful to broaden the clinical use of this combined approach looking at the simultaneous assessment of the anatomical and functional relevance of different coronary stenoses in patients with complex CAD. Further studies providing robust validation of this non-invasive technique are awaited.
European System for Cardiac Operative Risk Evaluation (EuroSCORE) II
The EuroSCORE II is a prognostic tool to predict the risk of in-hospital mortality in candidates for cardiac surgery. Anticipating the risk of surgery might be helpful to customise heart team indications and widen anatomical thresholds for percutaneous revascularisation when the operative risk becomes clinically unacceptable.
The EuroSCORE II encompasses 18 variables, including clinical, laboratory, echocardiographic and planned procedural parameters. Over recent decades, the score has been technically refined. The latest developed version of the EuroSCORE II showed better discrimination than former versions, namely the additive and logistic EuroSCORE models.12 All versions of the EuroSCORE are provided in an online calculator for clinical use, which is available at www.euroscore.org. The EuroSCORE II has been extensively validated in the literature. A recent meta-analysis, based on 22 studies and including 145,592 patients undergoing cardiac surgery, showed a good overall good performance in terms of both discrimination and accuracy.13
Society of Thoracic Surgeons Risk Score
The Society of Thoracic Surgeons (STS) calculator requires the collection of a more extensive array of clinical parameters (http://riskcalc.sts.org).
Mortality at 30 days is the primary outcome measure predicted by the score, but the STS database has also been used to derive a series of additional prognostic models for different clinical endpoints.4 The discrimination of the STS score for in-hospital mortality in the CABG-only group is good (Table 1).14
Acute coronary syndromes
TIMI Risk Score
The TIMI risk score is intended to predict 30-day mortality in patients with STEMI who are eligible for fibrinolytic therapy.15 The score was derived from the 14,114 patients enrolled in the InTIME II trial and validated in the TIMI 9 trial.3 The c-statistic values in the derivation and validation cohorts were 0.779 and 0.746 respectively, consistent with moderate discrimination.
However, fibrinolysis in Western countries has been largely replaced by primary PCI use. which nowadays is the revascularisation modality recommended by guidelines for patients with STEMI.
Nevertheless, the TIMI risk score still provides acceptable discrimination for the prediction of 1-year mortality (c-statistic 0.725) in patients treated with primary PCI.16,17
SIMPLE Risk Index
The SIMPLE risk index is a pragmatic, user-friendly score to predict the risk of 30-day mortality in patients with STEMI. The score is calculated by using three clinical variables which are routinely collected at the first medical contact, namely age, heart rate and systolic blood pressure.
The SIMPLE risk index was derived using data of patients enrolled in the InTIME II trial3 and externally validated in the TIMI 9A/B trial. Of note, the score is also a robust predictor of mortality occurring within 24 hours from symptoms onset (c-statistic=0.81).18
GRACE Risk Score
The GRACE risk score was derived from a large, multinational registry of patients with ACS (>20,000 patients). The score, which should be calculated at hospital admission, predicts the risk of mortality at six months. The c-statistics in the derivation and validation cohorts were 0.81 and 0.75 respectively (Table 1).19
The use of the GRACE risk score is advocated by current practice guidelines for the management of patients presenting with NSTEMI to stratify their clinical risk and select the optimal timing for revascularization.20 An online calculator of the score is available at http://gracescore.org/WebSite/Default.aspx.
ACTION–GWTG Model
This model, based on the large ACTION Registry-GWTG database, is intended to predict the risk of in-hospital mortality in patients admitted with acute MI. Importantly, patients presenting with cardiac arrest or cardiogenic shock, who have often been excluded during the derivation of other risk scores, are included in this model.21 The c-statistic value for the ACTION-GWTG model was remarkable in both the derivation and the validation cohorts (0.88) (Table 1).
Risk Scores After Treatment
In patients with CAD, therapeutic strategies aimed at counteracting and relieving ischaemia, the pathophysiological substrate of CAD, are key to sizably improve clinical outcomes either in the early phases after the index event and in the long term. Ignoring the benefits conveyed by effective treatment, such as those of multi-targeted medical therapy and revascularisation may lead to bias and inaccuracy in prediction and risk stratification. Taking this into account, different risk scores have been developed based on the treatment modality received during the index hospitalisation and by incorporating parameters reflecting the response to the treatment itself.
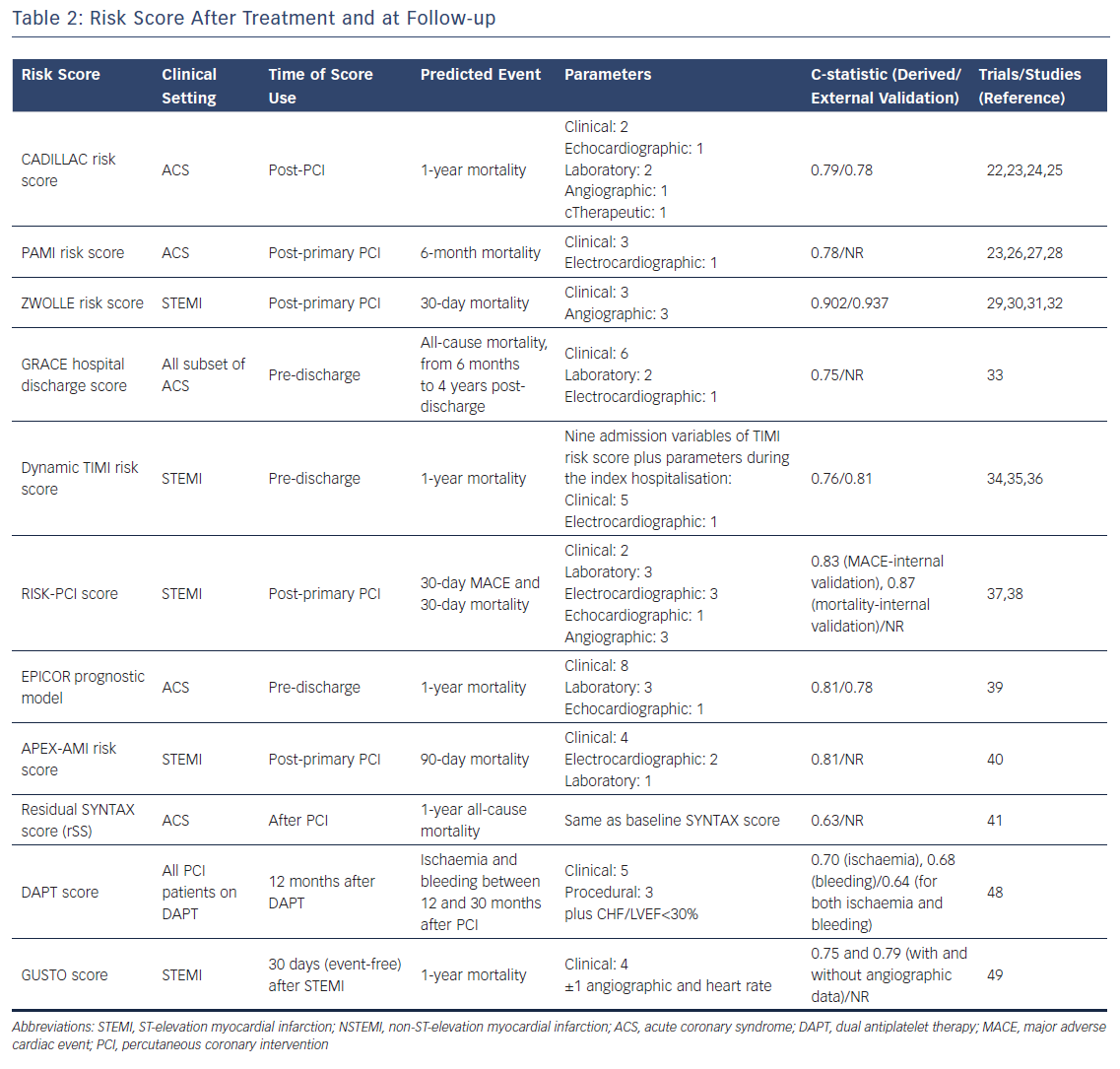
CADILLAC risk score
The CADILLAC risk score, which has been derived from patients included in the CADILLAC trial,22 aims at stratifying the risk of death at one year in patients with ACS undergoing revascularisation by PCI. The external validation of the score was assesed in patients enrolled in the Stent-PAMI trial23 (Table 2). The score, which includes age >65 years (2 points), Killip class 2/3 (3 points), baseline left ventricular ejection fraction <40 % (4 points), anaemia (2 points), renal insufficiency (3 points), triple-vessel disease (2 points), and post-procedural TIMI flow grade (2 points), has to be calculated in the immediate post-PCI setting. It has three classes of risk: low risk, score 0-2; intermediate risk, score 3-5; and high risk, score ≥6. The score showed good discrimination for predicting both 30-day and 1-year mortality with c-statistic values of 0.81 and 0.78 in the validation cohort, respectively.24
The main limitation of the CADILLAC risk score is the non-contemporary PCI strategy adopted in the trial. Patients in the CADILLAC trial were treated with bare metal stents which are no longer the default strategy during PCI. Therefore, the prognostic performance of the score may be inaccurate since drug-eluting stents (DES), the current standard of modern PCI practice, have demonstrated to significantly improve clinical outcomes in patients undergoing PCI.25
PAMI Risk Score
The PAMI risk score has been derived from a pooled analysis of the different PAMI trials, namely PAMI 1 and 2,26,27, AIR PAMI28 and STENT PAMI23 trials. The score has been designed to predict mortality at six months in patients with acute myocardial infarction treated with primary PCI. The discrimination ability of the score was fairly good (c-statistic 0.78) 3 (Table 2). The same limitations as the CADILLAC score (e.g. non-contemporary PCI practice) must be taken into account when using this score in clinical practice.
ZWOLLE Risk Score
The ZWOLLE risk score is simple, based on six clinical and angiographic variables (i.e. Killip class, post-procedural TIMI flow, age, three-vessel disease, anterior infarction and ischaemia >4 hours), which identifies STEMI patients treated by primary PCI at low risk (score ≤3) of 30-day mortality. This score could be used to select patients who can be safely discharged after 48-72 hours of having the procedure. In the validation cohort, the score was robust at predicting 30-day mortality (c-statistic=0.902).29 The predictive value of the ZWOLLE risk score has been confirmed in three randomized controlled trials.30–32
GRACE Score at Hospital Discharge
The GRACE score evaluated at hospital discharge to predict long-term mortality (i.e. beyond six months and up to four years) was validated in 1,057 hospital survivors included in the GRACE registry. The GRACE score was a robust predictor of all-cause mortality for all subsets of patients with ACS (STEMI, NSTEMI and unstable angina) at all analysed long-term follow-up time points (6 months, 1 year and yearly up to 4 years) with a c-index >0.75 at all evaluated time points.33
Dynamic TIMI risk score
The dynamic TIMI risk score estimates the risk of mortality at 1 year in STEMI patients at hospital discharge.34 The score was derived from the ExTRACT‐TIMI 25 trial35 (c-statistic in the derivation cohort of 0.76) and was subsequently validated in 3,534 patients with STEMI enrolled in the TRITON‐TIMI 38 trial36 (c-statistic in the validation cohort of 0.81).
The strength of this score is that it reclassifies risk based on the incidence of adverse events occurring during the index hospitalisation, namely recurrent MI, stroke, major bleed, heart failure/shock, arrhythmia or renal failure. The dynamic TIMI risk score is obtained by adding to the baseline TIMI risk score a series of weighted integer points related to the prognostic impact on mortality of each potential in-hospital adverse event. Thus, this model updates the baseline TIMI risk score with clinical determinants of subsequent prognosis. This process statistically translates into an improved prognostic performance of the dynamic TIMI risk score over the baseline TIMI risk score.
RISK-PCI score
The RISK–PCI score has been derived from a large cohort of patients treated with primary PCI (n=2,096) with the goal of predicting the risk of MACE and mortality at 30 days. The score – alongside clinical and laboratory parameters (i.e. age >75 years, prior infarction, anterior infarction, complete atrioventricular block, new-onset bundle branch block, left ventricular ejection fraction <40 %, high leucocytes count, glucose ≥6.6 mmol/L, creatinine clearance, pre-procedural TIMI flow = 0) – takes into account the results of the primary PCI procedure (post-procedure TIMI flow <3, reference vessel diameter ≤25 mm). It has shown good discrimination and calibration in the derivation cohort (the c-statistics in the internal validation cohort were 0.83 and 0.87 for MACE and mortality, respectively.37,38
The EPICOR prognostic model
Pocock et al. proposed a web-based, user-friendly risk score to predict the risk of two-year mortality in 23,489 consecutive hospital survivors after an ACS event included in EPICOR and EPICOR Asia prospective cohort studies.39 Twelve independent predictors of mortality were combined into the score, and a good discrimination was achieved (c-statistic 0.81).
Consistent with the dynamic TIMI risk score, the EPICOR model also accounts for the incidence of in-hospital adverse events; quality of life before discharge, as assessed by the EQ-5D questionnaire, is also used in the calculation of the score. An online web calculator of the EPICOR prognostic model is available at www.acsrisk.org.
APEX-AMI risk score
This APEX-AMI risk score is a prognostic model predicting the risk of mortality at 90 days in patients with STEMI undergoing primary PCI. The model was derived using data from patients enrolled in the APEX-AMI (Assessment of Pexelizumab in Acute Myocardial Infarction) trial. The model had good performance (c-statistic 0.82) and was internally validated, demonstrating a c-statistic value of 0.81.40
Residual SYNTAX score
The residual SYNTAX score (rSS) – calculated by subtracting the score of each successfully treated lesion from the baseline SYNTAX score – was firstly proposed by Généreux et al. in patients with moderate- or high-risk ACS undergoing PCI enrolled in the prospective ACUITY trial.41 The rSS is useful to numerically quantify the burden and complexity of residual CAD after PCI and a strong independent predictor of ischaemic outcomes at 1 year, including all-cause mortality, with a c-statistic 0.63.
The rSS has been extensively validated in the literature. Farooq et al. found that the rSS was a robust predictor of 5-year mortality in the SYNTAX trial (in eligible patients with 3-vessel or left main coronary artery disease undergoing PCI)42 whereas the authors’ group demonstrated the prognostic value of the rSS as an independent predictor of 2-year cardiac mortality in the setting of unprotected left main PCI.43 The rSS represented an independent predictor of major adverse cardiac and cerebrovascular events (MACCE) at 1 year in patients with STEMI and multivessel disease.44 In addition, the rSS has been shown to be a predictor of short-term adverse clinical outcomes (30–day and 6-month all-cause death) in patients with ACS complicated by cardiogenic shock45, while Khan et al. found that an rSS>8 was a predictor of in-hospital death, congestive heart failure, recurrent MI and bleeding in patients treated with primary PCI.
Risk Scores During Follow-Up
Patients with CAD, despite effective treatment according to current guideline recommendations, are at substantial risk of experiencing recurrent ischaemic adverse events.47 Tailored management of patients during follow-up, based on individual risk profiles, is advisable to customise clinical management strategies for secondary prevention. The use of risk stratification tools might also be useful in secondary prevention.
DAPT score
The DAPT score was derived from 11,648 patients enrolled in the DAPT trial and externally validated in the PROTECT trial. The score should be used after 12 months of uncomplicated DAPT, to assess more reliably the benefit-to-risk ratio of prolonging DAPT treatment.48
The DAPT score simultaneously evaluates and weights factors associated with increased risk of bleeding (moderate or severe bleeding according to the GUSTO criteria) and recurrent ischaemic events (stent thrombosis or myocardial infarction) at follow-up. The score ranges from –2 to 9 and patients with a DAPT score of ≥2 may most likely benefit from the prolongation of DAPT.
In the derivation cohort, the model had c-statistic values of 0.70 and 0.68 for predicting ischaemic and bleeding risk respectively; in the validation cohort, the c-statistic was 0.64 for both ischaemic and bleeding risk. A user-friendly online calculator of the DAPT score is available at http://tools.acc.org/DAPTriskapp.
The DAPT score has some limitations. In particular, stenting was mostly performed with first-generation DES in the DAPT trial, so the risk of stent thrombosis and/or ischaemic events may be overestimated by the DAPT score in light of the high safety standards for the newer generation DES used today.
GUSTO score
In the GUSTO trial, data of STEMI survivors was used to derive two algorithms for predicting 1–year mortality after a 30–day event-free period: one nomogram is based on only clinical parameters and its c-statistic is 0.70; the other nomogram integrates clinical and angiographic variables and it has a c-statistic value of 0.75.
However such models had poor validation in subsequent studies and their use nowadays is generally limited due to the larger proportion of patients treated with primary PCI.49
Stratification of Bleeding Risk
The clinical implementation of potent anti-thrombotic therapies in patients at heightened cardiovascular risk has come at the cost of increasing the risk of bleeding.
Despite thrombotic recurrences being the most feared events in patients with CAD, bleeding complications have a detrimental effect on prognosis which, ultimately, may offset the benefits of more intensive/prolonged pharmacological strategies for secondary prevention. Against this background, clinical research has been directed toward the stratification of bleeding risk in patients with CAD and different tools have been recently developed to support clinicians in the difficult clinical task of pinpointing the optimal balance between ischaemic and bleeding risk.
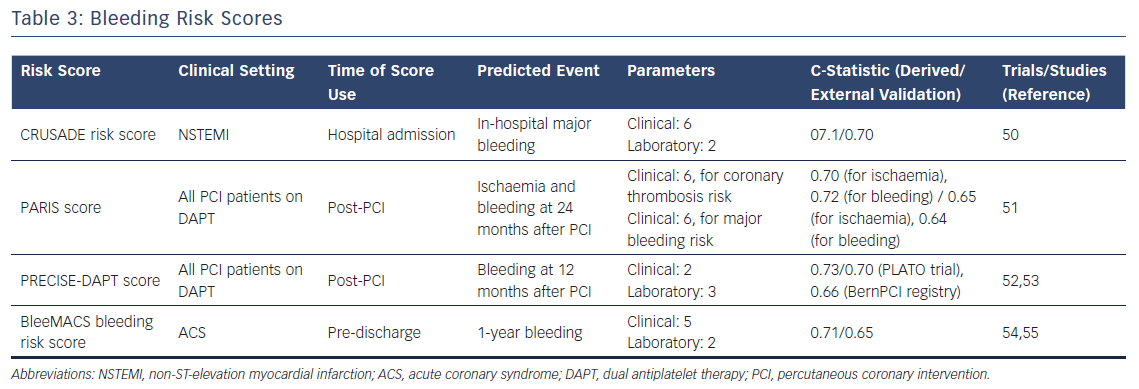
CRUSADE risk score
In patients with NSTEMI, the CRUSADE risk score is intended to predict the risk of major bleeding during the index hospitalisation. It was created by assigning a weighted integer value to each independent predictor of in-hospital bleeding: baseline hematocrit; glomerular filtration rate; sex; heart rate and systolic blood pressure on admission; prior vascular disease; diabetes mellitus; and signs of heart failure on admission.
The final score ranges from 1 to 100 points. Notably, the rate of major bleeding increased by bleeding risk score quintiles are: 3.1 % for score ≤20; 5.5 % for score 21–30; 8.6 % for score 31–40; 11.9 % for score 41–50; and 19.5 % for score >50. C-statistic values for the derivation and validation cohorts were 0.71 and 0.70, respectively.50 (Table 3).
PARIS risk score
The PARIS risk score is a simple and useful tool to predict the risk of ischaemic and bleeding events at two years after PCI with DES. In detail, the two separate scores were developed using data from the PARIS (Patterns of Non-Adherence to Anti-Platelet Regimen in Stented Patients) registry. The first model to predict ischaemic events includes six clinical variables (diabetes mellitus, ACS presentation, current smoking, creatinine clearance <60 ml/minute, prior PCI and prior CABG) with a c-statistic value of 0.70; the second model, predicting major bleeding, also has six clinical variables (age, body mass index, current smoking, anaemia, creatinine clearance <60 ml/min, triple antithrombotic therapy on discharge) with c-statistic of 0.72.
External validation was performed in the ADAPT-DES (Assessment of Dual Antiplatelet Therapy With Drug-Eluting Stents) registry and discrimination was moderate, with c-statistics of 0.65 and 0.64 for ischaemic and bleeding risk scores respectively.51
PRECISE–DAPT Score
The PRECISE-DAPT score was developed from a collaborative, individual patient-level analysis including data from eight randomised controlled trials (14,963 patients). The model predicts the risk of bleeding in patients treated with dual antiplatelet therapy (c-statistic of 0.73 for out-of-hospital TIMI major or minor bleeding and 0.71 for TIMI major bleeding within 12 months).52
The following variables are included in the score: age; creatinine clearance; hemoglobin; white blood cell count; and previous spontaneous bleeding. Patients at high bleeding risk are identified by a score of 25 or greater.
In the external validation, obtained in two independent PCI-treated populations from the PLATelet inhibition and patient Outcomes (PLATO) trial and the BernPCI Registry, the PRECISE-DAPT score showed c-statistic values of 0.70 and 0.66 respectively. The PRECISE-DAPT score showed improved integrated discrimination and reclassification performance compared with the PARIS score in both validation cohorts for TIMI major or minor bleeding.
The PRECISE-DAPT score, which can be easily calculated at www.precisedaptscore.com, is endorsed by the 2017 European Society of Cardiology guidelines on optimal DAPT duration in patients undergoing PCI.53
BleeMACS Bleeding Risk Score
The BleeMACS (Bleeding complications in a Multicenter registry of patients discharged with diagnosis of Acute Coronary Syndrome) registry is a multicentre retrospective registry that enrolled more than 15, 000 patients with ACS who were treated with PCI.54 The BleeMACS bleeding risk score was derived and internally validated in this registry, and demonstrated to be a simple tool for estimating the risk of post-discharge serious bleeding events up to one year.
The score includes seven predictors: age; hypertension; vascular disease; history of bleeding; malignancy; creatinine; and haemoglobin. The BleeMACS risk score exhibited good performance in the derivation (c-statistic: 0.71) and internal validation (c-statistic: 0.72) cohorts. The c-statistic in the external validation cohort, performed in the SWEDEHEART registry, was slightly lower (c-statistic of 0.65 for PCI-treated patients and 0.63 for patients who did not undergo PCI).55
Selecting a Risk Scoring System in Daily Clinical Practice
Undoubtedly, the marked increase in the number of risk scores available to interventional cardiologists, as well as the presence of overlapping risk scores in the same clinical scenarios, make it difficult to select the best risk scoring system in daily clinical practice.
In the growing arena of risk assessment tools, however, only a limited number of risk scoring systems have been extensively, rigorously and externally validated. This is a central issue in guiding the selection and supporting the rationale for the use of a specific risk score in daily clinical practice (Figure 2, bottom panel).
In patients with stable CAD, the SYNTAX I and II,7,9,56 the Euroscore II,12,13 and STS score4 have a central role in clinical decision making. Their applicability is firmly supported by the consistent and reproducible results regarding their overall performance and discrimination ability (as reported in Table 1).
In patients with NSTEMI, the GRACE risk score is particularly useful for the prediction of mid-term clinical outcomes and the identification of the optimal timing of myocardial revascularisation.19,20 In this group of patients, the CRUSADE risk score is also robust at predicting the risk of major in-hospital bleeding.50
The ACTION-GWTG model should be clinically implemented to assess the risk of in-hospital mortality in patients admitted with acute MI (it has excellent discrimination – see Table 1).21 After treatment for STEMI, the Zwolle risk score is instrumental at predicting the risk of early (30-day) mortality, thus allowing the selection of patients at low risk of adverse events who can be safely discharged within 72 hours of revascularisation by primary PCI.29–32
Finally, for the optimisation of medical therapy after PCI, the latest European guidelines recommend the use of the PRECISE–DAPT and DAPT scores (class of evidence IIb/A) to properly decide the optimal duration of dual antiplatelet therapy after PCI.53 Even though further research is crucial in this setting, clinicians should take advantage of these tools when facing the difficult clinical task of minimising the risk of bleeding while giving secondary prevention from recurrent ischemic events (Figure 2).
Advancing Risk Stratification in Patients with CAD: a Glimpse into the Future
Bedside prognostication using simple and broadly accessible clinical variables, coupled with experience, is the mainstay of risk stratification in medicine. Over recent decades, medicine has drastically evolved in parallel with the implementation and wider clinical use of more advanced diagnostic and therapeutic techniques.
Specifically, practice in cardiovascular medicine has been permeated by a large amount of additive information coming from more powerful diagnostic tools (i.e. CT of coronary arteries and invasive coronary diagnostic parameters), biomarker evaluation as well as proteomics and genomics. These data are complementary to the clinical evaluation and, undoubtedly, the integration of clinical and advanced diagnostic data have been instrumental to advance medical practice.
Nevertheless, prognostication remains challenging in specific clinical contexts such as cardiogenic shock. A complex interplay between haemodynamic, metabolic and clinical factors increase the complexity of risk stratification in these patients.
Recently, a promising risk score to predict 30-day mortality has been developed in this clinical setting using data from the landmark IABP-SHOCK II (Intraaortic Balloon Pump in Cardiogenic Shock) trial.57
In a new era of individualisation and precision in medicine, refinement of existing risk scores by newer diagnostic tools has proven to be of clinical value. As an example, the evaluation of the N–terminal pro–BNP was shown to improve the overall predictive performance of different risk scores (the. Zwolle, TIMI and GRACE risk scores) in patients with STEMI .58–60 Similarly, in patients with stable CAD, combining clinical variables and biomarkers into a unifying risk score showed potential for improving the stratification of risk for cardiovascular mortality.61 Moreover, the use of genetic risk scoring in patients with CAD has been recently proposed.62
Future studies will clarify if and how the use of these advanced techniques will advance our understanding of risk stratification in patients with CAD and if this process will finally translate into improved quality of care for PCI and ACS patients.
Conclusion
Different tools for risk stratification have been developed and validated in patients with CAD. A stepwise approach, considering the characterisation of both ischaemic and bleeding risk is advisable in these patients to better guide both the immediate and long-term medical management strategies.
Further studies are needed to clarify whether further improvements in risk stratification can be obtained by integrating the array of existing clinical risk scores with complementary information coming from more advanced diagnostic techniques.