Dr Burkhoff opened the meeting by reminding the attendees about the mission of the Acute Cardiac Unloading and REcovery (A-CURE) Working Group, which is to advance the science and mechanistic understanding of acute cardiac unloading and support the translation of basic and clinical research into therapies aimed at heart muscle recovery. He noted that the A-CURE symposium is the only scientific conference dedicated entirely to acute unloading and heart recovery, and clinicians have made great strides in accomplishing the mission.
He presented a brief history of the A-CURE meetings, which began with the first faculty meeting in Paris in 2015. The annual A-CURE symposium started in Rome in 2016, followed by Barcelona in 2017, Chicago in 2018 and Paris in 2019. He highlighted the increasing number of abstracts (31 in 2016 to 63 in 2019) and accelerating science over the years, including the initiation and completion of the ST-elevation MI Door-To-Unload (STEMI-DTU) pilot trial in 2017 to the expected commencement of the STEMI-DTU pivotal trial in 2019.
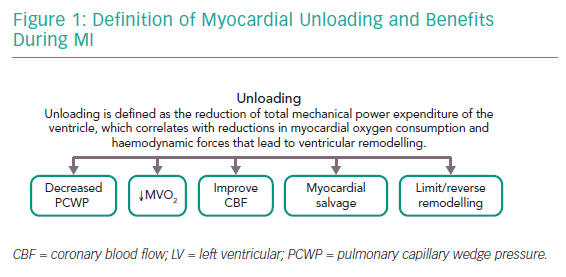
Dr Burkhoff proposed a formal definition of left ventricular (LV) unloading as the reduction of total mechanical power expenditure of the ventricle, which correlates with the reduction in myocardial oxygen consumption and haemodynamic forces that lead to ventricular remodelling.1 He explained the concepts of remodelling and reverse remodelling using pressure–volume loops (PVL), emphasising that the PVL is a graphical depiction of myocardial function that holds a wide range of physiologically relevant data. He highlighted that the PVL is bound by the end-systolic pressure–volume relationship and the end-diastolic pressure–volume relationship (EDPVR), indices of contractility and diastolic function, respectively. Following an acute insult to the myocardium, there is a reduction in contractility with a reduction in stroke volume and an increase in pulmonary capillary wedge pressure (PCWP). The sustained increase in haemodynamic load and neurohormonal activation leads to the initiation of ventricular remodelling.
Prolonged ventricular remodelling manifests as the rightward shift of the PVL representing haemodynamic stress and typically results in chronic heart failure. The goal of ventricular unloading is to prevent/reverse cardiac remodelling and subsequent heart failure. When the goal of myocardial unloading using mechanical circulatory support is achieved, it results in decreased PCWP, decreased myocardial oxygen consumption, improved subendocardial coronary blood flow, salvage of myocardium and limits/reverses remodelling (Figure 1).
He noted that the benefits of LV unloading are well-documented in basic and clinical literature. The seminal work by Pfeffer et al. in 1985 demonstrated the benefits of LV unloading using captopril, an angiotensin-converting enzyme (ACE) inhibitor, in a rat model of MI.2 They showed that during a large untreated MI, the EDPVR is markedly shifted rightwards compared to normal conditions, reflecting acute pressure and volume overload in the LV. However, the degree of EDPVR rightward shift is blunted following treatment with captopril. This pre-clinical finding was tested in a clinical trial that showed a significant reduction in end-diastolic pressure and PCWP with the resultant decrease in ventricular dilatation with captopril.3 These studies were followed by additional trials, such as the Survival and Ventricular Enlargement (SAVE) trial, that were critical in establishing ACE inhibitors as therapies for the treatment of MI.
However, there are inherent limitations to myocardial unloading using pharmacological therapies. Importantly, more unloading of the LV through pharmacological means leads to more compromise in aortic pressure and cardiac output. Hence, pharmacological therapies are inherently limited in their ability to unload the heart. On the other hand, the use of a percutaneous ventricular assist device, such as Impella, can simultaneously unload the ventricle and reduce the workload of the heart while increasing end-organ perfusion as perfusion pressure is maintained. In 2003, Meyns et al. demonstrated that LV unloading using Impella reduced myocardial oxygen consumption resulting in reduced infarct size in a sheep model of ischaemia–reperfusion.4 The study by Kapur et al. of a pig model of ischaemia–reperfusion injury further demonstrated that mechanical pre-conditioning with acute circulatory support before reperfusion limits infarct size in acute MI.5 These results led to the STEMI-DTU pilot trial in humans assessing the safety and feasibility of primary unloading with Impella followed by delayed reperfusion in patients with STEMI. The study confirmed the safety and feasibility of primary unloading, followed by delayed reperfusion, and showed that the infarct size was relatively independent of the area at risk. The results of the pilot study are the basis for moving forward with the STEMI-DTU pivotal trial, expected to start in December 2019.
Additional studies have shown that LV unloading in chronic heart failure can also induce reverse remodelling. Levin et al. showed that the EDPVR of hearts from patients with end-stage idiopathic cardiomyopathy were shifted rightwards towards markedly larger volumes compared to normal hearts.6 However, chronic haemodynamic unloading with LV assist devices resulted in the leftward shift of the EDPVR towards lower volumes, similar to those of normal hearts. Additional studies have demonstrated that the unloading-induced reverse remodelling in chronic heart failure is both dose- and time-dependent.7,8
Dr Burkhoff concluded that this year’s A-CURE symposium will showcase advances in the science of unloading and myocardial salvage from basic science and clinical research.