Over the last few decades, primary percutaneous coronary intervention (PCI) has revolutionised the treatment of ST-elevation myocardial infarction (STEMI) with rapid recanalisation of the infarct-related epicardial vessel, resulting in smaller infarct size and a substantial reduction in adverse clinical endpoints.1,2 However, suboptimal myocardial reperfusion is documented to occur in a relatively large proportion of patients undergoing primary PCI for STEMI, despite optimal restoration of epicardial flow, with unfavourable short- and long-term outcomes.3 STEMI patients are at particularly high risk of thrombus embolisation due to elevated thrombotic burden and prothrombotic milieu.4 Thrombus embolisation, either spontaneously or as a consequence of instrumentation, is associated with reduced levels of procedural success. While this is most commonly related to embolisation into the distal coronary tree, it also includes retrograde embolisation either into non-culprit vessels or systemic emboli, which could further complicate primary PCI.
Distal embolisation can lead to re-occlusion of the culprit vessel or its downstream branches and is a major contributor to slow and no re-flow by occlusion of distal microvasculature, leading to ongoing ischaemia despite a patent epicardial artery. Thereby, evidence of distal embolisation is quantified by thrombolysis in MI (TIMI) flow, myocardial blush grade and ST segment resolution. While angiographic signs of distal embolisation occur in 6–18% of cases of primary PCI in STEMI,5–11 the true incidence may be much higher, demonstrated by retrieval of visible debris in up to 73% patients in studies such as the Enhanced Myocardial Efficacy and Recovery by Aspiration of Liberated Debris (EMERALD) trial.12
Thrombus embolisation is associated with adverse procedural results and a greater frequency of adverse outcomes, including larger infarct size, reduced left ventricular ejection fraction, larger enzyme rises and increased rates of recurrent MI and mortality.5,6,13 A high thrombus burden has been associated with incidence of distal embolisation and in itself is associated with PCI failure and adverse outcome in STEMI.14–16 Due to the prognostic implication of thrombus embolisation, management of lesions with high thrombotic burden remains a challenge in the setting of primary PCI for STEMI.
Case
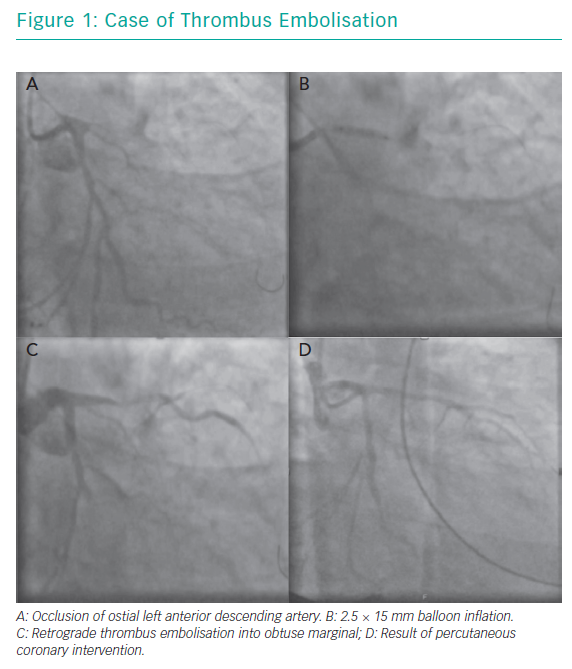
A 49-year-old man presented with a history of chest pain and worsening breathlessness over the previous 3 days. He had a history of hypertension and smoking. On arrival he had on-going chest pain and was in mild pulmonary oedema. He was Kilip class II with a systolic blood pressure of 120 mmHg. His ECG showed a late presenting anterior STEMI with Q waves and a bedside echocardiogram demonstrated moderate to severely impaired LV function with anterior wall hypokinesia. A coronary angiogram demonstrated a chronic occlusion of the right coronary artery with an ostial occlusion of the left anterior descending (LAD) artery (Figure 1A).
A standard workhorse wire was taken to the distal LAD with no restoration of flow. A 2.5 × 15 mm balloon was inflated at the site of occlusion (Figure 1B). The next image showed retrograde thrombus embolisation into a large obtuse marginal branch (Figure 1C). This was accompanied by a drop in blood pressure requiring IV metaraminol and rapid deterioration to pulseless electrical activity (PEA) arrest. Cardiopulmonary resuscitation was initiated, an AutoPulse® device applied, and the patient was intubated. A second wire was passed to the circumflex vessel and after predilatation a 3.0 × 28 mm drug-eluting stent (DES) was deployed with a further 3.0 × 38 mm DES deployed in the LAD. Despite TIMI 3 flow in both vessels (Figure 1D), there was no return of spontaneous circulation and resuscitation was discontinued after 38 cycles of CPR.
This case demonstrates retrograde thrombus embolisation into a non-culprit vessel with a large amount of myocardium at risk due to chronic occlusion of the right coronary artery. To our knowledge, there are only two other published case reports in the literature. However, the outcomes are poor. This case highlights some technical considerations hat could have been considered, including passage of a second wire to the circumflex artery, thrombus aspiration upfront (with deep intubation of the guide catheter), use of glycoprotein (GP) IIb/IIIa inhibitors, and supportive measures, such as use of mechanical support either upfront due to the large area of myocardium at risk or at the point of thrombus embolisation; however, these can often be overlooked with the need for rapid restoration of TIMI 3 flow in the culprit vessel.
Thrombus embolisation, both distal and retrograde, is associated with increased morbidity and mortality in primary PCI and its management is still debated. Here we review current data for pharmacological and interventional strategies to prevent thrombus embolisation and suggest an optimal therapeutic strategy in the setting of large thrombus burden in primary PCI.
Angiographic Predictors of Thrombus Embolisation in Primary PCI
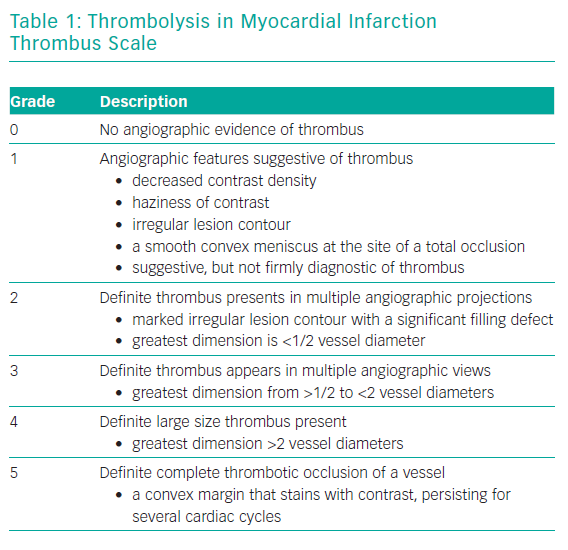
The main predictor of embolisation is thrombus burden. Thrombus burden may be classified angiographically using the TIMI thrombus grade in Table 1.17 Since there is a high incidence of coronary occlusion in STEMI and large thrombus burden, in this setting thrombus grade 5 is reclassified after wire crossing and balloon passage/inflation.14 Other predictors of embolisation include thrombus composition, TIMI flow, lesion length and large vessel diameter.5–7,18 The anatomical risk of embolisation should also be assessed before deciding on therapeutic strategy. This allows for adjunctive therapies, such as mechanical support (intra-aortic balloon pump, Impella [Abiomed], extracoroporeal membrane oxygenation) to be considered where large areas of myocardium are at risk or if large thrombus is located close to an important bifurcation; protecting both branches with wires may help to prevent occlusion and facilitate treatment in the event of thrombus embolisation. Assessment of thrombus burden and composition, and anatomical risk of embolisation in patients with STEMI undergoing primary PCI may optimise percutaneous treatment of these highly thrombotic lesions, guiding utilisation of pharmacological agents or interventional strategies, to reduce thrombus burden and improve both epicardial and myocardial perfusion.
Pharmacological Strategies in Prevention of Thrombus Embolisation
P2Y12 Inhibitors
All STEMI guidelines have recommended early upstream administration of oral P2Y12 inhibitors or at the latest at the time of PCI given their delay in onset of action.19 However, this is not evidenced by randomised trials. Upstream oral antiplatelet absorption may not always be achievable in situations of intubated patients or in patients with delayed absorption (e.g. morphine) or if associated vomiting. The only evidence currently available is from the 30 Day Study to Evaluate Efficacy and Safety of Pre-hospital vs In-hospital Initiation of Ticagrelor Therapy in STEMI Patients Planned for PCI (ATLANTIC) trial, which compared the upstream prehospital and periprocedural administration of ticagrelor in 1,862 STEMI patients with a mean time difference of 31 minutes. There was no difference in the rates of ST segment resolution or TIMI 3 flow between the two groups.20 Given that less than 50% patients with prasugrel and ticgarelor have optimal platelet inhibition at 2 hours and the problems with gastric absorption in specific patient groups anecdotally, these cases may be covered with GPIIb/IIIa inhibition.21 Recent guidelines recommend that cangrelor may be considered in patients who have not received P2Y12 inhibitors (IIb/A).19 Cangrelor is administered intravenously and has a faster onset of action. While there are no specific randomised trials in STEMI, pooled analysis from STEMI patients included in the Clinical Trial to Demonstrate the Efficacy of Cangrelor in PCI (CHAMPION PCI) and Clinical Trial Comparing Cangrelor to Clopidogrel Standard Therapy in Subjects Who Require PCI (CHAMPION-PHOENIX) showed a significant reduction in stent thrombosis at 30 days.22
Glycoprotein Inhibitors
Despite some evidence to suggest that adjunctive administration of GPIIb/IIIa inhibitors in STEMI may reduce mortality and re-infarction23 there is no evidence to suggest benefit over risk of bleeding with routine facilitated use of GPIIb/IIIa inhibitors in STEMI.24–28 Although initial non-randomised trials suggested benefit of localised intracoronary delivery of GPIIb/IIIa inhibitor over intravenous delivery in terms of TIMI flow and short-term mortality, no overall mortality benefit has been demonstrated.29 There also appears to be a signal for use of intracoronary GPIIb/IIIa use from theIntracoronary Abciximab and Aspiration Thrombectomy in Patients With Large Anterior MI (INFUSE-AMI) trial where abciximab was delivered using the ClearWay™ (Atrium Medical) infusion catheter where there was a reduction in infarct size in the intracoronary arm compared with intravenous of 2.3%, but this did not reach significance.30
However, the larger Abciximab IV Versus IC in ST-elevation MI (AIDA STEMI) trial (n=2065) did not show any reduction in clinical endpoints by intracoronary abciximab administration and this again was demonstrated in a more recent meta-analysis.31,32 Current guidelines suggest that while there is no evidence to recommend routine use of GP IIb/IIIa inhibitors, they may be considered in the event of angiographic evidence of large thrombus, slow- or no-reflow, and other thrombotic complications. It is important to note that this strategy has not yet been tested in randomised trials.
Anticoagulant Therapy
While there has been no randomised trial assessing unfractionated intraprocedural heparin in primary PCI there is a substantial body of experience and its use is recommended in current guidance (I/C).19 There have been some randomised trials evaluating bivalirudin in the setting of STEMI with a recent meta-analysis demonstrating no mortality advantage and concerns over excess rates of early stent thrombosis in the bivalirudin treated arms.25,33–37 Further, the most recent large randomised study on the impact of bivalirudin during primary PCI for STEMI, Bivalirudin versus Heparin in ST-Segment and Non-ST-Segment Elevation MI in Patients on Modern Antiplatelet Therapy in the Swedish Web System for Enhancement and Development of Evidence-based Care in Heart Disease Evaluated according to Recommended Therapies Registry (VALIDATE-SWEDEHEART) trial randomising 3,005 patients with STEMI showed no benefit conferred by bivalirudin use in rates of myocardial infarction, bleeding and death at 180 days compared to unfractionated heparin.38 Therefore, current guidance has downgraded recommendation of bivalirudin use from I/B preferred to IIa/A (consider). Bivalirudin is also recommended in heparin-induced thrombocytopaenia.19
Intracoronary Thrombolysis
Early small studies of local delivery of fibrinolytics as an adjunct to primary PCI showed promising results with improvement in myocardial reperfusion and TIMI flow in the infarct-related artery, suggesting that this may be beneficial adjunctive treatment in STEMI patients at high risk of thrombus embolisation, without the known systemic adverse effects.39,40 The first randomised trial, Delivery of Thrombolytics Before Thrombectomy in Patients With ST-Segment Elevation Myocardial Infarction Undergoing primary Percutaneous Coronary Intervention [DISSOLUTION]), where 102 patients with STEMI and large thrombus burden were randomised to either intracoronary urokinase delivered by microcatheter or placebo prior to thrombectomy demonstrated increased rate of TIMI 3 flow, myocardial blush grade, ST segment resolution as well as improvement in 6-month major adverse cardiac events.41 However, the recently reported Trial of Low-dose Adjunctive alTeplase During prIMary PCI (T-TIME), which randomised 440 patients with STEMI to either placebo, 10 mg alteplase or 20 mg alteplase after the first device in primary PCI (37–42% use of thrombectomy), showed no difference in the primary endpoint of extent of microvascular obstruction on cardiac MRI at 2–7 days.42 There are currently two ongoing Phase III trials to evaluate intracoronary low-dose alteplase, the Adjunctive Low-dose tPA in Primary PCI for STEMI (STRIVE, NCT03335839) study, and tenecteplase, the Restoring Microcirculatory Perfusion in STEMI (RESTORE-MI; ACTRN12618000778280) trial.
Mechanical Strategies in Prevention of Thrombus Embolisation
Thrombectomy
Thrombectomy devices have been developed in an attempt to prevent thrombotic complications in STEMI by reducing thrombus burden and thereby enhancing the benefits of primary PCI. These have been evaluated for routine use in STEMI in some studies.
Mechanical devices include the AngioJet® (MEDRAD), X-SIZER® System (Covidien) and Rinspiration (eV3) that actively fragment and aspirate thrombus, and the TVAC® (Nipro) and Rescue (Boston Scientific) that aspirate thrombus only without fragmentation. Several trials using different mechanical devices have led to conflicting results. Both the AngioJet Rheolytic Thrombectomy In Patients Undergoing Primary Angioplasty for Acute MI (AIMI) trial randomising 480 STEMI patients to thrombectomy with Angiojet versus standard primary PCI and a second study randomising 215 patients to thrombectomy with the Rescue catheter versus standard primary PCI paradoxically demonstrated large infarct sizes in the treated arms.43,44 Conversely, the AngioJet Rheolytic Thrombectomy Before Direct Infarct Artery Stenting in Patients Undergoing Primary PCI for Acute MI (JETSTENT) trial where the Angiojet was tested in a cohort with large thrombus burden showed significant improvements in ST segment resolution, and 6-month major adverse cardiac event despite no difference in infarct size.45 A meta-analysis of mechanical thrombectomy in STEMI showed no improvement in reperfusion or mortality despite a benefit in ST segment resolution.46
Most of the current available thrombectomy data pertains to manual thrombus aspiration using the Export® Aspiration Catheter (Medtronic) or the Diver® (Invatec). These devices are simpler to use compared to mechanical devices. However, they are limited by the inability to aspirate large amounts of thrombus rendering them theoretically less effective in reducing thrombus load.
Manual thrombectomy devices have shown benefits compared with standard primary PCI using surrogate endpoints, such as TIMI flow, ST segment resolution, myocardial blush grade, infarct size and LV function.47 However, evidence regarding clinical endpoints such as re-infarction, mortality and MACE as well as concerns regarding safety have meant that current guidance has downgraded the use of routine thrombectomy from recommended (IIa/B) to not recommended (III/A).19
The Thrombus Aspiration during Percutaneous coronary intervention in Acute MI Study (TAPAS) trial, where more than 1,000 STEMI patients were randomised to either routine thrombus aspiration (Export Aspiration Catheter) or conventional primary PCI, showed significant improvement in myocardial blush grade and ST segment resolution as well as increased 1-year survival.9
However, recently, two large-scale randomised trials of manual powered to evaluate hard clinical endpoints comparing routine manual thrombus aspiration with standard primary PCI have definitely demonstrated no beneficial effect of routine thrombectomy.48,49 The Thrombus Aspiration During ST-Segment Elevation Myocardial Infarction in Scandinavia (TASTE) trial recruited over 7,000 patients and showed no difference in mortality between the two groups at 30 days or 1 year.48,50 The Trial of Routine Aspiration Thrombectomy With PCI Versus PCI Alone in Patients With STEMI (TOTAL) trial with over 10,000 patients corroborated this result with no difference in cardiovascular death, MI, cardiogenic shock, or heart failure at 1 year, despite improvements in surrogate markers of rate of distal embolisation and ST segment resolution.49,51 Importantly, TOTAL showed a clear safety signal with an increase in incidence of stroke at 1 year in the thrombectomy arm compared with primary PCI alone.52 The mechanism of this is presumed to be proximal or systemic embolisation of thrombus via the extraction of the device and it is therefore recommended that the guide be deeply engaged into the coronary ostium during thrombus aspiration and device removal as a preventative strategy; alternatively, a guide catheter extension may be used.
A recent meta-analysis combined data from 18,306 patients from the TAPAS, TASTE and TOTAL trials showed overall no differences between the two treatment groups in terms of mortality at 30 days, or incidence of stroke.53 Subgroup analysis demonstrated that in patients with the highest thrombus burden defined by TIMI thrombus grade ≥3 there were fewer cardiovascular deaths as well; however, this group also had increased incidence of stroke/transient ischaemic attack; this could suggest that if systemic embolisation could be prevented then benefit could be seen in this high thrombus burden subgroup of patients.
Taken together, this would suggest that patients with a high thrombus burden benefit the most from thrombectomy by reducing the incidence of distal embolisation. Current guidelines have suggested that while routine thrombus aspiration is not recommended, it may be considered in specific cases where there is a high thrombus burden and risk of embolisation.19 Innovations in device technology should now focus on mitigating risk of systemic embolisation and stroke during thrombectomy. Further trials are required to evaluate the utility and benefit of thrombus aspiration in cohorts with large thrombus burden; however, this would require large patient numbers to be powered to detect differences in hard clinical outcomes.
Embolic Protection Devices
Embolic protection devices include both proximal and distal devices. Distal devices are either occlusive, such as the PercoSurge (Medtronic) whereby an occlusive balloon is inflated distal to the lesion with debris aspirated prior to deflation or filters such as the FilterWire EZ (Boston Scientific), which is a non-occlusive, filter-based distal protection device. While there is evidence to support embolic protection devices in the prevention of thrombus embolisation vein graft PCI, these have not been replicated in the setting of STEMI. Both the Exploring the MEchanism of Plaque Rupture in Acute Coronary Syndrome Using Coronary CT Angiography and computationaL Fluid Dynamic (EMERALD) trial and ASPiration of Liberated Debris in Acute MI with GUardWire Plus System (ASPARAGUS) trials comparing adjunctive PercoSurge to conventional primary PCI in 501 and 341 patients, respectively, demonstrated safety of the devices but failed to show benefit in terms of infarct size.12,54 These results were reflected in the PROspective Multicenter Imaging Study for Evaluation of chest pain (PROMISE) trial which randomised 200 patients with STEMI to the FilterWire EZ or conventional primary PCI.55 An analysis of all trials of distal protection devices involving 1,353 patients showed that while there was some benefit in terms of myocardial blush grade there was no improvement in 30-day mortality.56
The only studied proximal protection device is the Proxis Embolic Protection System (Velocimed) which theoretically confers complete protection from distal embolisation by deployment proximal to the lesion interrupting antegrade flow and therefore has the benefit of protecting all distal branches while thrombus is aspirated. The PRoximal Embolic Protection in Acute MI and Resolution of ST-Elevation trial, the only trial to date evaluating this device in STEMI, showed no benefit conferred in either surrogate or clinical outcomes.57 Considering the paucity of data for embolic protection devices, they have not been recommended in current practice guidelines.
Excimer Laser Coronary Atherectomy
Excimer laser coronary atherectomy (ELCA) is potentially effective in reducing the size of coronary thrombus by inducing shock waves that can separate thrombus from the vessel wall, dissolve clot by acoustic waves within the thrombus structure and vaporise pro-coagulant mediators.58,59 Laser also has an inhibitory effect on platelet aggregation due to interaction with the 308-nm ultraviolet beam leading to ‘stunned platelet phenomenon’.60 Despite the theoretical potential, there are limited data to support its use in primary PCI. The Cohort of Acute Revascularization of Myocardial infarction with Excimer Laser (CARMEL) multicentre registry, enrolled 151 AMI patients, 65% of whom had large thrombus burden in the culprit artery who gained most benefit.61 TIMI grade flow was significantly improved and there was a low rate of major adverse cardiac event (8.6%). Recently, another large registry (Utility of Laser for Transcatheter Atherectomy-Multicenter Analysis around Naniwa [ULTRAMAN]) has reported on 175 STEMI patients treated with ELCA, again showing similar TIMI flow improvement, a 92.8% success rate, and major adverse cardiac event rate of 3.3%.62 The only randomised trial reported to date is the Excimer Laser Versus Manual Thrombus Aspiration in Acute Myocardial Infarction (LASER-AMI) trial where 27 STEMI patients were randomised to adjunctive ELCA or conventional primary PCI demonstrating safety of the device and similar outcomes in terms of TIMI grade flow, and myocardial blush in both groups.63
Stenting Strategy
A direct stenting strategy reduces the incidence of distal embolism and no-reflow in cases of high thrombus burden by trapping thrombus behind the stent. It has been validated by several studies that have demonstrated superiority over angioplasty with predilatation and stenting. In Harmonizing Outcomes with Revascularization and Stents in Acute MI (HORIZON-AMI) trial, the reperfusion indices (ST resolution, TIMI 3 flow, and no-reflow incidence) were better and the 1-year mortality was lower with this technique.64 However, the anticipated benefits need to be balanced alongside possible risks with this technique, including problematic stent delivery, incomplete lesion preparation and inaccurate stent sizing secondary to poor visualisation and vasoconstriction.
In terms of stent choice in cases of large thrombus burden, the MGuard™ (InspireMD) and STENTYS™ (STENTYS SA) stents have recently been evaluated in randomised trials. The MGuard is a covered stent with a bare metal stent platform with a fine outer mesh with the aim to trap thrombus without prolapse through stent struts and prevent thrombus embolisation. The MASTER I trial randomising 433 STEMI patients to either MGuard or conventional stenting demonstrated promising results with improvement in ST segment resolution and improved mortality at 1 year.65,66 However, the MASTER II trial, which aimed to recruit 1,114 patients, was terminated early due to a high rate of stent dislodgement.
The STENTYS is a self-apposing and self-expanding nitinol stent with its design offering several benefits in thrombus management. First, as the maximal expansion diameters of each of the stent sizes is at least 1 mm greater than the balloon size, a smaller diameter may be chosen than that of the artery, enabling a gentler and atraumatic deployment which limits the direct mechanical fragmentation of the atherothrombotic material. Second, the tight mesh allows better retention of the thrombotic mass against the wall. Third, the risk of late malapposition is reduced as expansion may continue with the stent conforming to the shape of the vessel as vasodilatation and thrombus lysis occur. This stent has been evaluated in a series of studies (APPOSITION IV).67–70 While use of this stent has not been studied in cases of large thrombus burden, APPOSITION IV randomised 152 STEMI patients to STENTYS or to conventional DES and reported a rate of malapposition at 4 months less with the STENTYS than with the comparator.70 Despite this, their clinical effectiveness in comparison to other stent designs still needs to be confirmed in large-scale, randomised clinical trials.
Deferred stenting implantation in primary PCI is also an option to reduce embolisation of thrombotic material in the presence of high thrombus burden once antegrade flow has been restored. This can allow 24–48 hours of intense antithrombotic therapy, including prolonged intravenous GPIIb/IIIa inhibition. Subsequent angiography frequently shows reduced thrombus burden such that PCI may be performed with a significantly lower risk of distal embolisation.71 While the randomised Deferred Stenting Versus Immediate Stenting to Prevent No- or Slow-Reflow in Acute ST-Segment Elevation Myocardial Infarction (DEFER-STEMI) trial showed significantly lower rates of no-reflow in a high risk population,72 the MIMI trials showed no difference in rate of microvascular obstruction seen on cardiac MRI between immediate invasive treatment compared with deferred PCI.73 This signal was confirmed in the larger Deferred Versus Conventional Stent Implantation in Patients with ST-segment Elevation Myocardial Infarction (DANAMI 3-DEFER) study in which deferred stenting 48 hours after the index procedure had no effect on a composite clinical outcome of mortality and revascularisation of non-culprit vessels. However, it did demonstrate a higher rate of target vessel revascularisation.74 On the basis of the available data, deferred stenting is not recommended in the current guidelines (III/A).19
Therapeutic Strategy for Prevention of Thrombus Embolisation in Primary PCI
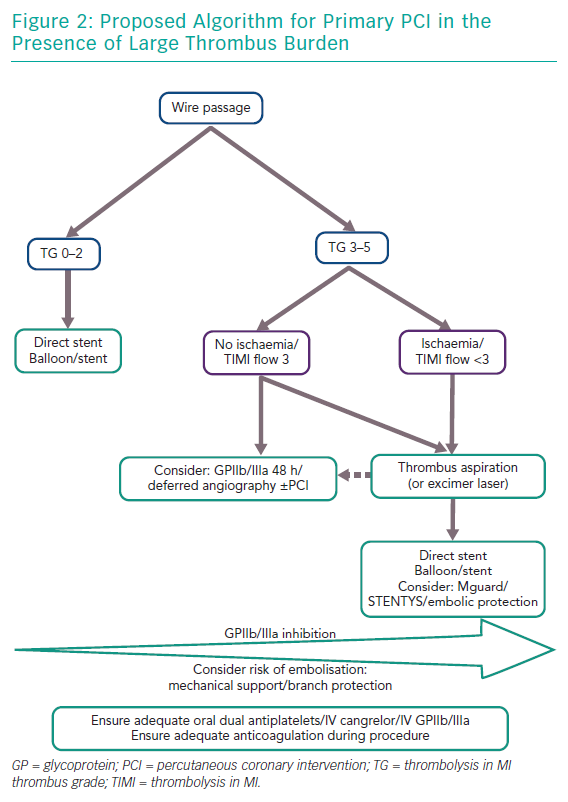
There is conflicting evidence as to the ideal management strategy in cases of large thrombus burden in STEMI. Based on the evidence presented here we propose an interventional algorithm in these cases (Figure 2). For all cases, potent antiplatelet activity must be ensured to minimise the risk of thrombus expansion and new thrombus formation including consideration as to gastric absorption of oral aspirin/P2Y12 agents. If there is any doubt in this the patient may be covered with either IV cangrelor or GPIIb/IIIa inhibition upfront. Furthermore, anticoagulation should be maintained with either heparin or bivalirudin. Often wire crossing or balloon passage without inflation can restore flow. Assessment of thrombus burden may then be made using the TIMI thrombus grading.14 As thrombus grade increases more benefit may be yielded from administration of GP IIb/IIIa inhibition. Further, consideration should be given to anatomical risk of thrombus aspiration in terms of need for supportive measures such as mechanical support as well as protection of branches especially in ostial stenosis. If the TIMI thrombus grade is ≥3 as described as large thrombus burden,14 aspiration thrombectomy should be considered and multiple runs may be necessary (or excimer laser if available although this is based on limited evidence). Thrombus aspiration if performed should either be with deep guide catheter engagement or a guide catheter extension to decrease risk of retrograde embolisation. Moreover, suction should be maintained until the thrombectomy catheter is removed from the guide catheter. Thereafter, balloon angioplasty and stenting may be performed, although there may be benefit in the setting of large thrombus load for direct stenting and consideration of either the MGuard or STENTYS stents. If antegrade flow is restored and large thrombus burden remains without ongoing ischaemia one other option to consider in preventing thrombus embolisation would be to defer further intervention by 24–48 hours with intense antithrombotic therapy, including prolonged GPIIb/IIIa inhibition.
Conclusion
Large thrombus burden in STEMI can further complicate primary PCI due to spontaneous or mechanical embolisation, either distally or retrograde, into a non-culprit vessel or systemically. In the context of no proven recommendation in this setting, we discuss some adjunctive and preventative pharmacological and interventional strategies and propose a management algorithm in the primary PCI setting.