Patients with multivalvular disease (MVD) can present with a variety of valve pathologies and combinations, often requiring a complex treatment strategy. Both the Euro Heart Survey and the EURObservational Research Programme Valvular Heart Disease Registry (presented at the European Society of Cardiology [ESC] 2018 meeting) demonstrated that more than one-fifth of patients with native valve disease have MVD.1 As these registries solely report left-sided MVD, it seems safe to assume that the number of patients with multiple right- and left-sided valve disease is even higher. Data on the treatment and outcome of such patients is scarce. As most trials and studies concentrate on single-valve pathologies, the MVD patient cohort is also underrepresented in the 2017 ESC/European Association for Cardio-Thoracic Surgery (EACTS) guidelines for the management of valvular heart disease, even more so regarding interventional treatment options.2
This article provides an insight into percutaneous treatment possibilities for this heterogeneous patient population.
Evaluation
The clinical presentation of patients with MVD depends on various interacting factors, including the severity of each single valve lesion, the type of lesion (insufficiency or stenosis), volume status, ventricular function and the time of clinical presentation. Furthermore, patient comorbidities and level of activity can have an effect on symptoms. Auscultation and identification of murmurs can be difficult. In patients with MVD of similar severity, the clinical symptoms may be masked by the haemodynamic effects of the most proximal lesion. A thorough and extensive examination, as well as a comprehensive patient history, is mandatory in order to better understand the leading pathology.
In addition, ECG is an essential diagnostic tool for valve evaluation. Yet, most quantification methods have been validated for single-valve disease and so their use in MVD may not yield correct results.
Nonetheless, ECG assessment should include quantitative measurements of valve dynamics, a description of valve morphology and functional indices, such as ventricular function and dimensions and pulmonary pressure.3 In general, measurements that are not affected by volume status, such as planimetric dimensions or measurement of the vena contracta, are preferred.2,3 Further information regarding valve pathology and relation to cardiac structures can be obtained using 3D ECG. Valve pathology, in particular the difference between primary or secondary mitral or TR should be clearly described, because this affects the type of intervention, surgical treatment and possible improvement after treatment of other valves. It must be kept in mind that valve pathologies influence one another, masking or exaggerating the true severity of each lesion. For example, a severely insufficient mitral valve may cause flow reduction across the aortic valve, leading to a decreased gradient, and volume overload due to heart failure in severe aortic stenosis may cause mitral regurgitation. The differentiation can be difficult.
Invasive cardiac catheterisation for pressure and cardiac output measurement should remain restricted to patients in whom non-invasive tests are inconclusive or show a discrepancy with clinical findings.2
Treatment
Data about the treatment of MVD are scarce. The 2017 ESC/EACTS guidelines provide only general suggestions as opposed to evidence-based recommendations for the treatment of patients with MVD.2 In general, recommendations for the treatment of concomitant valvular disease are primarily only Level C recommendations. Briefly, treatment should follow the recommendations for the dominant lesion; and interactions between valve lesions and the higher surgical risk for multiple valve interventions must always be taken into account, including the likelihood of spontaneous changes in tricuspid and mitral regurgitation after surgery.2
As a key principle, treatment of patients with MVD should be performed by a multidisciplinary heart team in a specialised heart valve centre.2,4 The heart team should include surgeons, interventionalists, echocardiologists, anaesthesiologists and radiologists. The combination of expertise from each discipline will allow for the best possible care of the patient. The rationale for treating patients in a heart valve centre is that such centres provide the logistics and high volume needed to minimise complication rates.4
In the setting of patients with MVD, not only is the timing of the intervention important, but even more so is the type of intervention. In the Euro Heart Survey, in-hospital mortality for treatment of MVD was 6.5%, compared with 0.9–3.9% for single-valve disease.1 In the Society of Thoracic Surgeons database, the operative mortality of patients with MVD was twice as high as for patients with single-valve disease (10.7% versus 5.7%, respectively; p=0.0001).5 Furthermore, long-term mortality and, in particular, valve-related mortality in patients undergoing aortic and mitral valve replacement are high.6,7 This higher risk must be taken into account when evaluating patients and deciding on the treatment strategy. The possibility of a single-valve operation as an incomplete surgical correction in order to reduce the risk for selected patients should be taken into account. Conversely, a percutaneous intervention may be a lower-risk single-valve treatment.
In summary, data and recommendations regarding treatment of MVD are scarce and underrepresented. The guidelines primarily include surgical treatment options and advise concomitant interventional valve procedures because of the invasiveness of surgery. Although interventional valve treatment options have, to date, only been established for selected and single-valve disease (e.g. transcatheter aortic valve replacement [TAVR] and MitraClip [Abbott Vascular]), less-invasive and possibly staged procedures may play a crucial role in the treatment of patients with MVD. This also provides the possibility of symptomatically treating multimorbid patients, addressing only the dominant valve lesion. By staging interventional procedures, it is possible to treat one lesion and to then re-evaluate the severity of coexisting valvular pathologies in a ‘normalised’ haemodynamic situation.
Percutaneous Interventional Devices Beyond Transcatheter Aortic Valve Replacement and MitraClip
TAVR and MitraClip are well-established procedures that will not be described in detail in this article. Numerous studies have proven that the use of TAVR as a valve-in-valve (ViV) procedure in the aortic position can be performed with excellent results.8,9 The clinical use of transcatheter prostheses has also been expanded to include mitral ViV, mitral/tricuspid valve-in-ring (ViR) and valve-in-mitral annular calcification (ViMAC). A recently published study showed excellent results for mitral ViV, despite a high-risk population.10 However, in that study, mitral ViR and ViMAC were associated with a higher rate of adverse events and mid-term mortality than ViV.10 Aside from various case reports, the use of transcatheter prostheses in the tricuspid position has only recently been published. In this high-risk population, mortality was lower than expected and valve dysfunction or thrombosis was uncommon.11 Further studies will help us understand the applicability of this procedure. The sections below describe a small selection of percutaneous devices beyond the spectrum of the known TAVR valves and MitraClip. The diversity and evolution of interventional devices will allow for better patient-adapted therapies, in particular for patients with MVD.
Percutaneous Mitral Valve Devices
Aside from the MitraClip, which addresses leaflet coaptation, various transcatheter devices mimicking known surgical strategies have been developed. These include, but are not limited to, the NeoChord DS1000 (NeoChord,) for neochordae implantation, the Cardioband (Edwards Lifesciences) and the Mitralign Percutaneous Annuloplasty System and the Carillon Mitral Contour System (Cardiac Dimension) for mitral valve annuloplasty.
The NeoChord DS1000 is a minimally invasive, beating-heart transcatheter system developed for echo-guided neochordae implantation via a transapical access. Procedural and short-term safety and efficacy have been demonstrated, although long-term results are sparse.12
The Cardioband system is a transcatheter direct annuloplasty device, leaning on the known surgical technique of a restrictive annuloplasty. Results from a feasibility trial and early clinical experience are promising.13,14 At the moment, the Annular Reduction for Transcatheter Treatment of Insufficient Mitral Valve (ACTIVE; NCT03016975) trial is on-going in the US. This is a prospective multicentre randomised controlled and pivotal trial, comparing transcatheter mitral valve repair using the Cardioband system together with medical treatment compared to medical treatment alone in patients with functional MR. The study will randomise 375 patients in a 2 : 1 randomisation scheme.15
The Mitralign Percutaneous Annuloplasty System is a direct annuloplasty technique that applies sutures anterior and posterior of the commissures. By cinching these sutures, annular reduction is achieved. During the first-in-human trial, the device success rate was 70%, with postoperative left ventricular remodelling and improved clinical status.16 Larger series and longer follow-up have not been reported as yet.
The Carillon Mitral Contour System is an indirect annuloplasty device that is inserted in the coronary sinus. Mitral valve leaflet coaptation is achieved by septal–lateral compression of the posterior annulus. During the Carillon Mitral Annuloplasty Device European Union Study (AMADEUS), the device was only successfully implanted in 30 of 48 patients because of access issues, high residual MR and problems with coronary artery blood flow.17 Subsequent trials (i.e. Transcatheter Implantation of Carillon Mitral Annuloplasty Device [TITAN] and TITAN II) using the second generation device) showed higher device success, a low major adverse event rate and good left ventricular remodelling.18,19 Again, long-term data and larger case series are still needed.
Transcatheter Mitral Valve Replacement
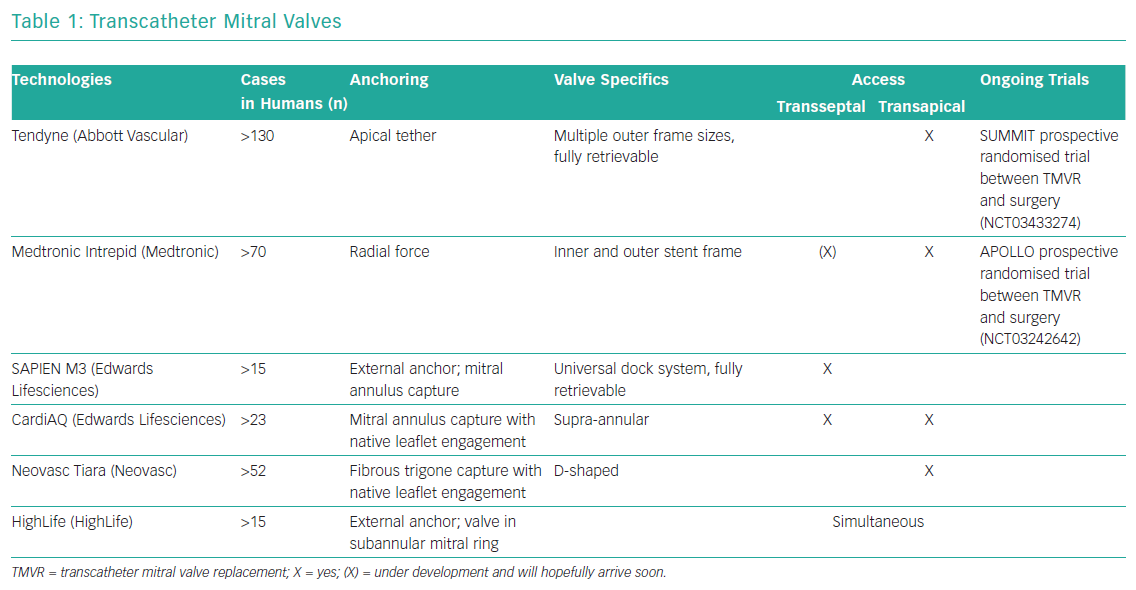
Development of transcatheter mitral valve devices has proven to be a greater challenge than that of aortic valve devices. The main challenge lies in the complex anatomy of the mitral valve and its relationship to other anatomical structures. This includes the asymmetrical shape of the mitral annulus, large leaflets, in most cases a lack of calcified structures and the risk of left ventricular outflow tract obstruction. Access and anchoring are challenging. Table 1 gives a brief overview of the most established transcatheter mitral valves with no claim to completeness. At the moment, there is no transcatheter mitral valve replacement with CE marking available.
Percutaneous Tricuspid Valve Devices
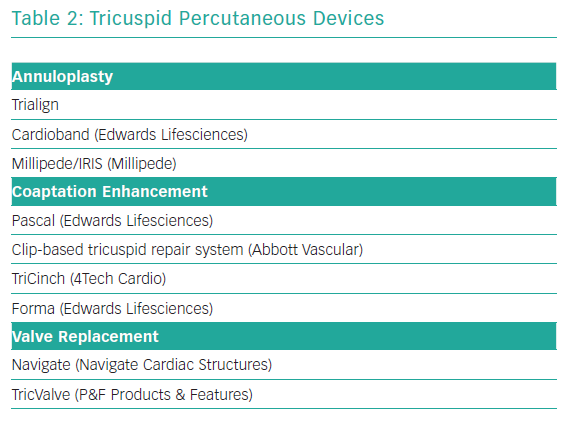
Transcatheter tricuspid valve treatment solutions have recently been emerging. To date, most of devices have been used in small case series or compassionate human case series. Most common has been the off-label use of the MitraClip (see below). As with the mitral valve, dedicated tricuspid valve devices focus on mimicking surgical techniques, such as annuloplasty, leaflet coadaptation or valve replacement. Table 2 gives an overview of this investigational group of devices, again with no claim to completeness.
Common Concomitant Valve Pathologies
Aortic Valve Stenosis and Mitral Valve Regurgitation
Aortic stenosis will lead to remodelling and hypertrophy of the left ventricle, as well as left ventricular dilation. Although primary mitral regurgitation can be present, the changes in left ventricular geometry can lead to secondary mitral regurgitation. Concomitant moderate to severe mitral valve regurgitation (MR) in patients undergoing TAVR has been reported to occur in approximately 20–30% of patients and has been shown to have a significant effect on mortality.20–22 In most trials, a significant improvement in MR was reported in 50–60% of patients after TAVR alone.20–22 The results of a large meta-analysis, including primary and secondary MR, indicate that the use of a balloon-expanding valve seems to have a greater effect on MR reduction than the use of a self-expanding valve.22 Functional MR and the absence of pulmonary hypertension, as well as the absence of AF, had positive effects on MR reduction after TAVR.23 As residual MR after TAVR has a negative effect on mortality, patients should be followed closely and, if necessary, undergo mitral valve intervention.
The type of intervention depends on valve pathology and the applicable devices. A possible percutaneous approach for the treatment of aortic valve stenosis and concomitant MR is TAVR followed by MitraClip implantation. Case series have been able to show the feasibility of this approach, yet rehospitalisation rates and midterm mortality are high, with low functional improvement.24,25 A 2017 study with data from the German Transcatheter Mitral Valve Interventions (TRAMI) registry showed a lower device success rate and a lower survival rate for patients who had previously undergone surgical or interventional aortic valve replacement (AVR; n=28).26 In the TAVR subgroup, survival at 1 year was <50%.26 These results suggest that, at present, this percutaneous treatment combination may be limited to a high-risk subgroup, although larger prospective trials must be established. A further possible percutaneous approach is the combination of TAVR and the Neochord DS1000, which was reported by Gerosa et al. to yield good results.27 Additional data are required to show the feasibility and safety of this approach.
Aortic Stenosis and Mitral Valve Stenosis
The combination of aortic stenosis and mitral stenosis is uncommon in industrialised countries because the haemodynamic situation is not well tolerated and patients present before severe stenosis becomes apparent. The presence of double-valve severe stenosis, leading to low cardiac output, will lower the respective gradients and thus lead to an underestimation of the severity of the stenosis. Here again, valve pathology is of great importance because rheumatic and degenerative mitral stenosis are addressed differently. Rheumatic mitral stenosis, caused by commissural fusion, can be well treated with percutaneous balloon valvuloplasty. Combined with an underestimated severe aortic stenosis, the sudden increase in preload may lead to heart failure with pulmonary oedema. Conversely, degenerative mitral stenosis is usually caused by progressive annular calcification.28 Treatment options, including surgery, can be difficult. As mentioned above, ViMAC is an option, but is still undergoing clinical testing.10
Aortic Valve Regurgitation and Mitral Valve Regurgitation
Aortic and mitral regurgitation is poorly tolerated, due to severe volume overload, leading to eccentric left ventricular hypertrophy and a reduction in function.29 MR due to volume overload caused by aortic regurgitation is not uncommon. Moderate to severe mitral regurgitation in patients undergoing AVR for aortic regurgitation has a negative effect on survival; simultaneous mitral valve repair improved survival.30 Staged percutaneous interventions allow for reassessment of mitral regurgitation and left ventricular function after treatment of aortic valve regurgitation. Taking certain anatomical criteria into consideration, aortic valve regurgitation can be treated using TAVR devices developed for aortic valve stenosis. A more than moderate paravalvular leak, the need for a second valve due to aortic regurgitation or valve embolisation and a higher rate of pacemaker implantation than usual for TAVR must be taken into account.31 An on-going study of a transfemoral TAVR valve specifically developed for aortic regurgitation will expand therapeutic options.
Left-sided Valve Disease and Tricuspid Regurgitation
Significant tricuspid regurgitation (TR) is prevalent in 5–30% of patients undergoing left heart surgery.32 Persistent TR after aortic or mitral valve surgery or intervention has been shown to have a negative effect on survival.33–35 Data concerning improvement of TR after TAVR is variable, with studies reporting TR improvement in 15–50% of patients.32,36 TR has been shown to be significantly reduced after MitraClip implantation, but persistent moderate or severe TR independently predicted death and rehospitalisation for heart failure at 12 months.37 Although data concerning the effect of TR on outcomes for patients undergoing left heart surgery are very diverse, it seems clear that residual regurgitation has a negative effect on outcome.
Data from the Transcatheter Tricuspid Valve Therapies (TriValve) Registry show that 30% of tricuspid interventions are performed concomitantly with mitral valve intervention, and that 7.5% of patients had previously undergone left heart transcatheter intervention.38 In the largest series on tricuspid clipping, Nickenig et al. reported that 33% of patients undergoing tricuspid valve clipping using the MitraClip had previous interventional valve treatment of the aortic or mitral valve (19% TAVR, 14% MitraClip).39 Twenty-two patients (34%) underwent concomitant mitral valve clipping, and there was a similar reduction in TR and no difference in procedural complications between patients undergoing tricuspid clipping alone and concomitant mitral valve clipping.39 Hypothetically, the combination of TAVR or MitraClip with an interventional device for the tricuspid valve is possible and, with the poor outcomes of residual TR in mind, may be a reasonable treatment option. Further trials and development of transcatheter devices for the tricuspid valve must prove clinical feasibility and efficacy.
Conclusion
Patients with MVD are common and present with heterogeneous valve pathologies. Evaluation is complicated by interactions of the various valve pathologies. Trials and guidelines focus primarily on single-valve disease, providing few recommendations for the treatment of patients with MVD. Therefore, a summary of principles is presented below for the diagnosis and intervention of these patients based on our own clinical experience and the sparse available data:
- Evaluation should include an extensive clinical examination, a thorough anamnesis and a comprehensive echocardiographic analysis while taking the effects of the various valve pathologies into account.
- Patient risk assessment should be done in a heart team setting, respecting patient comorbidities and treatment goals (complete correction, improvement of quality of life).
- To date, surgical strategies have been the gold standard, yet interventional treatment modalities may offer various advantages, such as being less invasive, staged procedures and solely symptom oriented.
- For percutaneous interventions, the dominant lesion should be addressed first. After a recompensation period, re-evaluation of remaining valve pathologies and the clinical status of the patient is required. Data regarding the outcome of various interventional procedures (e.g. for the tricuspid valve) and, in particular, staged interventions are limited, therefore these treatment strategies should, at present, be used in inoperable or high-risk patients.